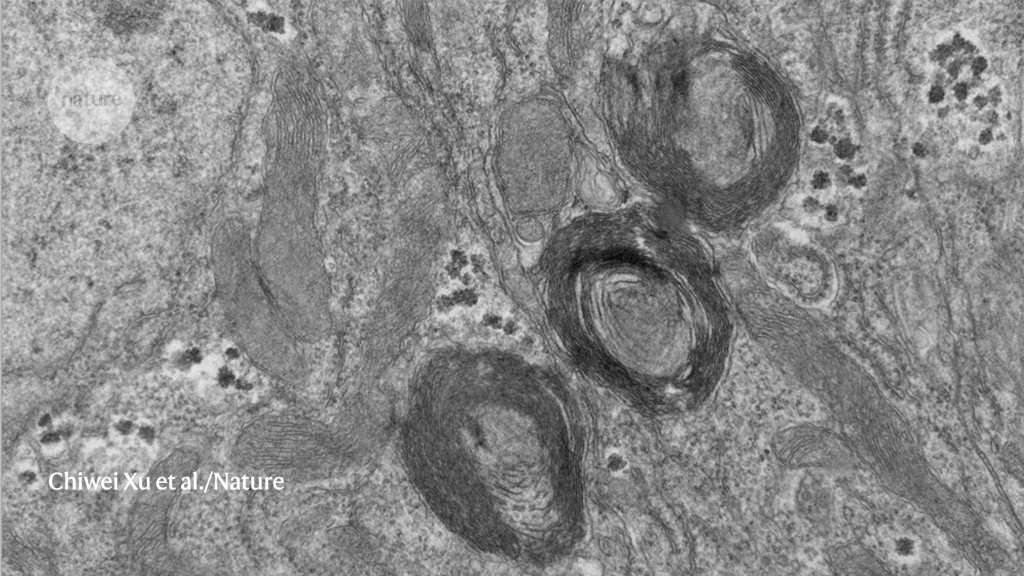
Three of the newly discovered organelles in a fruit fly intestinal cell. The organelles appear to be a reservoir of phosphate, a molecule essential to life.Chiwei Xu et al./Nature
Phosphate is essential to life. Now, researchers have discovered a tiny structure inside animal cells that acts like a reservoir of phosphate, helping to regulate levels of the nutrient inside cells and triggering processes that maintain tissues when it is in short supply1. The researchers classify the structure as a new type of organelle — fundamental structures within cells, such as the nucleus, mitochondria and membrane that function as a miniature organs within its body.
“This is one of the first studies to actually find phosphate storage in an animal cell,” says Rebekka Wild, a structural biologist at the French national research agency CNRS in Grenoble, who was not involved in the research. “It’s really exciting.”
In plants, bacteria and yeast, phosphate is important for cell growth and helps cells to communicate and generate energy. While it is known to be essential in animal tissues and cells, few studies had explored its specific functions. Charles Xu, a geneticist at the Rockefeller University in New York City, was curious what role phosphate played in regulating tissue renewal in the fruit fly gut, a useful model for studying how diseases affect cells in the human intestine. “That’s not really well known, especially in animal cells,” says Xu.
Fruit fly findings
Xu and his colleagues fed fruit flies (Drosophila melanogaster) phosphonoformic acid (PFA), which inhibits absorption of phosphorous in cells. When the researchers stained and imaged cells from the flies’ intestinal lining, they noticed that the lack of phosphate led to a spike in cell numbers. This rapid cell multiplication also occurred when Xu and his colleagues fed the flies food that contained 10% less phosphate than standard levels, indicating that the phosphate did indeed have an impact on cell numbers.
To find out how phosphate was having this effect, Xu and his team investigated whether low phosphate levels affected gene expression. A gene that the authors call PXo is similar to a mammalian gene that encodes a phosphate-sensing protein. Xu and colleagues found that PXo‘s expression was weaker when cells were deprived of phosphate. This reduced gene expression also kicked cell division into overdrive. However, cell division slowed down when the researchers tweaked the gene to overexpress the PXo protein.
The researchers labelled the PXo protein with a fluorescent tag and noticed that it was associated with an array of oval-shaped structures in the cells that did not seem to be any of the known organelles.
Phospholipid reservoirs
“These were quite visible, and we wondered what they were,” says Xu. When the scientists took a closer look at the mysterious structures, they saw they had several membrane layers, and the PXo protein was transporting phosphate across them. Once inside the unfamiliar organelles, the phosphate was converted to phospholipids, the main building blocks of cellular membranes.
When the fly cells were deprived of phosphate, the organelles broke apart and released the stored phospholipids into each cell, indicating that they function like reservoirs, says Xu. This breakdown activated cellular machinery known as Cka, triggering a stress signal that increased the production of new cells. This could be a way for the intestinal lining to keep phosphate levels stable, because the increased number of cells can absorb more of the nutrient, says Xu. “It’s beneficial for the organism to regenerate more of these healthy [cells],” he says.
Wild says that the findings lay the groundwork for exploring whether there are similar phosphate-storing organelles in other animals, including humans. She adds that it could be useful to take a deeper look at the structure of the PXo protein, to unravel how it transports phosphate into the organelles. “This would be very interesting, especially for people who come from the structural-biology side,” she says.
Xu says a next step could be to investigate how these phosphate-storing organelles interact with other organelles, and how their dynamics change over time. “It’s opened the door to many other questions,” he says.
The discovery of a new organelle in animal cells also highlights how much there is still to learn about cell physiology, adds Xu. “The beauty is there, it’s just waiting for us to discover it,” he says.


More News
The Amazon’s gargantuan gardeners: manatees
Publisher Correction: Single-crystalline metal-oxide dielectrics for top-gate 2D transistors – Nature
The baseless stat that could be harming Indigenous conservation efforts