Ageing is a key driver of bone-mass reductions and skeletal fragility. The bone loss that occurs with ageing reflects the confluence of many molecular and cellular processes, and it has therefore been more difficult to understand than the mechanistically distinct form of bone loss associated with the decline of oestrogen in women after menopause1–3. However, insights into the identity of skeletal stem cells (SSCs) and other related progenitor cell populations that produce bone-forming cells called osteoblasts4,5 have facilitated investigation into how ageing affects skeletal cells. Writing in Nature, Ambrosi et al.6 now determine how, with ageing, the function of SSCs changes, contributing to bone loss and impaired skeletal regeneration.
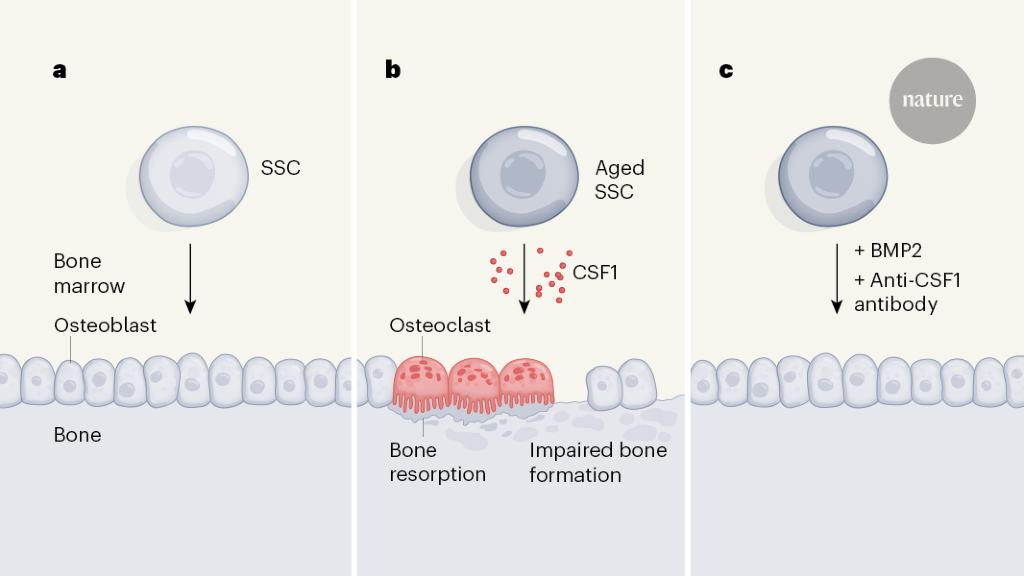
To distinguish the effects of intrinsic ageing-driven changes in these stem cells from environmentally driven changes, Ambrosi et al. isolated SSCs from the bones of young (2-month-old) and aged (24-month-old) mice and transplanted them into young recipient mice, in which the transplants formed small masses of bone tissue. This approach revealed two key differences between young and aged SSCs (Fig. 1a, b). First, the bone mass produced by aged SSCs was much smaller than that produced by young SSCs. Second, aged SSCs exhibited an increased ability to promote the formation of osteoclasts, the blood-derived cell type responsible for bone resorption. Thus, ageing hobbles the ability of SSCs to maintain a healthy balance between driving bone formation and bone destruction.
Next, the degree to which the aged environment affects SSCs was examined. The authors surgically joined old and young mice, thereby placing old and young SSCs in a shared blood circulation. In line with aged SSCs retaining functional deficits after transplantation, this approach did relatively little overall to normalize bone formation in the aged mice. Therefore, the ageing-driven reprogramming of SSCs seems to be a function of the direct effects of ageing on SSCs, rather than being driven by ageing-associated factors circulating in the blood.
Given that a young circulatory environment did not seem to reverse SSC ageing, efforts to counteract SSC ageing might be best targeted towards the effects of ageing on the downstream functions of SSCs. By measuring gene expression in aged SSCs, the authors found that colony stimulating factor 1 (CSF1), a soluble protein that promotes osteoclast maturation, is secreted at increased levels by aged SSCs compared with young SSCs.
Accordingly, the authors tested a therapeutic strategy to address SSC ageing. They mixed antibody molecules that specifically bind and block the function of CSF1 together with BMP2 — a protein with complex effects that can include the promotion of bone formation7 — into a gel, which they placed around bone fractures in young and aged mice. Remarkably, the combination of BMP2 and anti-CSF1 antibody treatment reversed the deficits in fracture healing observed in aged mice (Fig. 1c).
Overall, this report clearly establishes that ageing directly affects SSCs and that ageing-associated deficits in SSCs contribute to skeletal deterioration over time. However, it remains unclear to what degree these ageing-driven deficits specifically reflect changes in the stem-cell functions of these SSCs, such as self-renewal, as opposed to changes in how these cells differentiate or in the function of the mature osteoblasts they produce.
As one of the first studies that defines the basis of skeletal ageing in terms of specific and well-characterized stem-cell populations, this report serves as a powerful starting point for future mechanistic studies, including those clarifying how specific skeletal cell types participate in ageing-associated bone loss. In particular, parallel studies in mice have demonstrated that a population of bone-marrow-resident skeletal cells expressing the proteins CXCL12, EBF3 and LEPR account for a progressively increasing fraction of bone-forming cells with ageing8–10. How this population relates to the stem cells in Ambrosi and colleagues’ study remains unclear. Clarifying this point will be crucial to piecing together the broader cellular basis of skeletal ageing.
Skeletal ageing also encompasses a broader range of changes beyond the effects explained here, including the accumulation of senescent cells, increases in marrow fat content and changes in articular cartilage and bone architecture that include an overall widening of bones11. Future work will need to evaluate which of this broader set of ageing-associated changes are specifically due to intrinsic changes in SSCs, and which reflect the impact of other mechanisms of ageing.
Furthermore, definitions of the cell types that make up bone, including SSCs, are rapidly becoming more precise, and it is likely that an iterative process of refining these definitions will continue over the next few years. Indeed, a study published in February indicated that certain combinations of protein markers used by some researchers to define SSCs can also be expressed by mature bone-forming cells12. This suggests that extra markers will be needed to define SSCs as a pure population of stem cells. Our understanding of SSC-based mechanisms of ageing will need to keep pace as the definitions of skeletal cell types continue to evolve.
Competing Interests
The authors declare no competing interests.




More News
Karaoke-related stress soars after a good night of REM sleep
AI & robotics briefing: AI decodes languages in first ‘bilingual’ brain-reading device
Mexico’s next president is likely to be this scientist — but researchers are split in their support