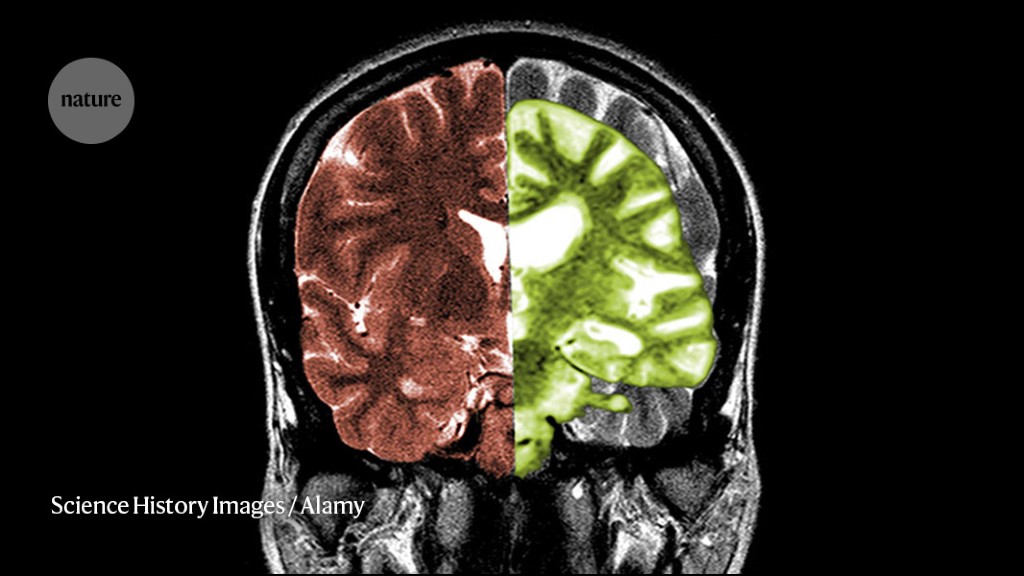
Alzheimer’s disease, as shown on the right side of this scan, transforms the brains of people with the neurodegenerative condition.Credit: Science History Images/Alamy
A scientific journal has revamped its whistle-blower policy amid a dispute over the integrity of research underlying an experimental Alzheimer’s drug.
In a 1 November 2022 editorial in The Journal of Clinical Investigation (JCI), editor-in-chief Elizabeth McNally wrote that whistle-blowers had raised concerns in August 2022 about potentially doctored images in research papers published by multiple journals, including JCI. Some of the papers were related to the experimental drug simufilam, developed by biopharmaceutical firm Cassava Sciences, based in Austin, Texas, whereas others were authored by a scientist involved in the treatment’s early testing.
Could drugs prevent Alzheimer’s? These trials aim to find out
But, McNally says, the whistle-blowers did not disclose conflicts of interest when they lodged their complaint — and she alleges that they have profited from short-selling Cassava stock, a practice in which individuals bet that a company’s stock will fall, and make money when it does. “This represents a new means of manipulating the scientific-publishing industry,” she writes.
Typically, when a whistle-blower contacts a journal about concerns over manipulated images or otherwise questionable data, the allegations are taken on good faith, McNally told Nature. The idea that whistle-blowers could be doing this for their own financial gain “was very eye-opening to me”, she says.
The group of four whistle-blowers who complained to JCI and other journals deny any wrongdoing and stand by their allegations, with three saying that they made only relatively small amounts of money from trading Cassava stock. When writing to the journals, they flagged possible image manipulation in 32 papers published by Hoau-Yan Wang, a medical researcher at the City University of New York (CUNY) who was responsible for much of simufilam’s preclinical testing. (Five of the papers were published by Springer Nature, which also publishes Nature; Nature’s news team is independent of its publisher.)
“Our so-called conflicts of interest also have absolutely nothing to do with the objective facts in play, which are manipulated images and data,” says Adrian Heilbut, one of the whistle-blowers, who is a computational biologist and independent consultant based in New York City. Cassava denies any wrongdoing.
A drug-development controversy
The company says that simufilam, a small-molecule drug, combats Alzheimer’s by stabilizing a crucial scaffolding protein in the brain called filamin-A that is impaired by the disease. Other Alzheimer’s drugs use monoclonal antibodies to target clumped amyloid proteins for removal from the brains of people with the disease.
FDA approves Alzheimer’s drug lecanemab amid safety concerns
Amid the allegations about Cassava’s data, researchers have expressed concern over how simufilam works. Aside from the preliminary studies by Cassava and its collaborators, the strategy of stablilizing filamin-A to tackle Alzheimer’s hasn’t been on anyone’s radar, says George Perry, an Alzheimer’s researcher at the University of Texas at San Antonio. “The fact that it hasn’t been widely studied means that it hasn’t been confirmed.”
In a statement, a lawyer acting on behalf of Cassava told Nature: “The scientific community is rethinking the causes of Alzheimer’s disease and looking to alternative explanations and potential solutions.” Last year, two research groups, not affiliated with Cassava, published papers looking at the role that filamin-A could have in Alzheimer’s disease1,2, the statement adds.
Cassava reported interim data from a clinical trial of simufilam in a September 2021 press release. Fifty people with Alzheimer’s who were treated with the small molecule for 12 months had improved cognitive test scores from when they started the trial, according to the release. The trial was ‘open label’, however, meaning that participants knew they were receiving the treatment, with no placebo group to ensure the improvement was caused by simufilam.
This is how an Alzheimer’s gene ravages the brain
A month before the company released these results, a pair of short sellers independent of the whistle-blowers who contacted JCI filed a petition with the US Food and Drug Administration (FDA), pointing to potential image manipulation in Cassava’s simufilam research and asking the agency to halt clinical trials. Cassava stock was at a high of US$135 per share before the petition was filed; shortly afterwards, the stock’s value had plummeted by 55%.
The US Securities and Exchange Commission is investigating, according to media reports. But the FDA announced in February 2022 that it would not intervene in Cassava’s clinical trials because the petition was not an appropriate way to request that the agency take this action.
The company has said that such ‘citizen-petition’ accusations are unsubstantiated and points out that “no government agency has accused Cassava Sciences of any type of dishonest behaviour”.
Investigation ongoing
At least five papers authored by Wang and others have been retracted over concerns about image manipulation, and other investigations are ongoing. So far, journals that published two3,4 of the three key research papers supporting simufilam as a potential Alzheimer’s treatment have issued expressions of concern and corrections. These journals told Nature that they have not closed the cases because they are waiting for the results of a CUNY investigation into Wang’s work. Cassava disputes this for one of the journals. In the case of the other, the journal says that it conducted its own investigation and found no compelling evidence of data manipulation with the intent to mislead.
Dozens of papers co-authored by Nobel laureate raise concerns
The third key paper, published in The Journal of Prevention of Alzheimer’s Disease5, was investigated, but one of its editors told Nature that it also found “no convincing evidence of manipulation of data or intent to mislead”, and therefore took no action. (This journal is published by Springer Nature.)
A spokesperson for CUNY told Nature that the university takes allegations of research misconduct seriously and cannot comment until its investigation is complete. Wang did not reply to Nature’s request for comment.
Last month, Cassava filed a lawsuit against the FDA petitioners, an investment company and the four whistle-blowers who contacted JCI: Heilbut; Jesse Brodkin, a pharmacologist who owns a preclinical-research equipment development company based in Basking Ridge, New Jersey; Enea Milioris, an independent portfolio manager who runs a life-sciences investment firm in London; and Patrick Markey, a clinical psychologist at an outpatient mental-health centre in Munich, Germany. Cassava says in its filing that the defendants spread “factually inaccurate and defamatory information”.
Heilbut and Markey told Nature that they had no comment. Brodkin and Milioris say that instead of providing evidence to counter their claims, the company is attacking them.
Policy change
McNally says she hopes that her JCI editorial will remind other journals to be aware of conflicts of interest. (JCI’s investigation of the allegedly doctored image in its paper did not substantiate the whistle-blowers’ claim, she says.) “Going forward, whistleblowers, just like authors, editors, and referees, will be asked to inform us of recent, ongoing, and potential conflicts of interest,” she wrote in her editorial.
Landmark Alzheimer’s drug approval confounds research community
One journal has already said that it might follow McNally’s lead. Louk Vanderschuren, editor of Behavioural Pharmacology, says that the journal will “definitely consider asking for disclosure of a financial conflict of interest, should an allegation of misconduct be reported to us” in future. The publication investigated allegations about one of the papers by Wang that has been scrutinized, but took no action because it received a satisfactory response from Wang, Vanderschuren told Nature.
Heilbut responds to McNally’s action, saying he and other whistle-blowers in his group disclosed their financial positions online. Brodkin tells Nature that although he has no problems declaring conflicts of interest, he worries about requiring disclosures from whistle-blowers. In some cases, he says, “whistle-blowers should be granted special protections against reprisal, including an option to raise concerns anonymously”.
Cassava began two phase III clinical trials in October and November last year, in which it plans to test simufilam on about 1,750 people.







More News
US funders to tighten oversight of controversial ‘gain-of-function’ research
Bird flu in US cows: where will it end?
Daily briefing: Why exercise is good for us