At first, it was impossible to tell them apart — all 83 mice in Catherine Belzung’s laboratory looked alike. But under stressful conditions, the differences between them grew stark. Some of the animals’ coats started to look shabby as they stopped taking care of themselves. They barely spruced up their nests, either — the same kinds of behaviour seen in people with depression.
By contrast, the other mice acted less stressed and they navigated a tricky maze with ease, says Belzung, a neuroscientist at the University of Tours, France. What was the difference? The more buoyant mice had a gene deleted and the drug tamoxifen injected, which spurred extra cell growth in the brain’s hippocampus, an area involved in mood regulation and memory.
The study1 offered fresh support for a theory that’s been gaining traction for years: that increased neuron formation, or neurogenesis, can prevent depressive symptoms from emerging — or reverse symptoms once they’ve begun. In some research, jump-starting neural growth seems to be just as effective at improving mood and motivation as popular antidepressant drugs such as selective serotonin reuptake inhibitors (SSRIs).
Fresh ways of stimulating brain-cell growth and connectivity “might result in a rethinking of the way we treat depression”, says neuroscientist Paul Albert at the University of Ottawa in Canada. Future therapies that encourage cell growth and development might be ideal for people with depression who do not respond to existing treatments. Neurogenesis studies are also identifying biological pathways that hint at how older treatments, such as SSRIs and exercise, spur cell growth in the brain. But before dedicated cell-growth therapies can reach the clinic, researchers need to fill in more details about the precise biological events that trigger neural growth — and the best ways to ensure new cells integrate with the brain’s existing neural networks.
A delicate balance
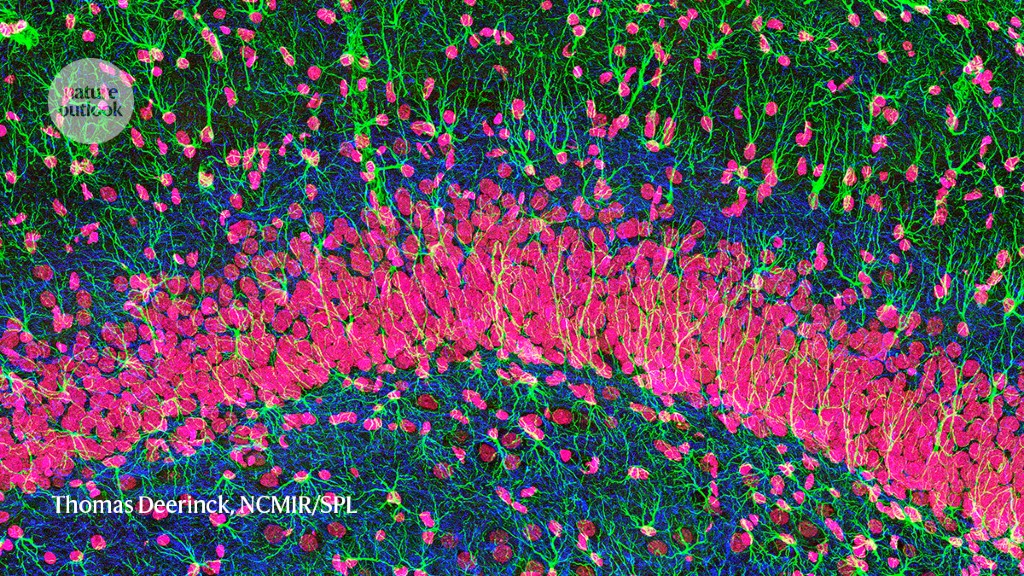
For decades, scientists thought that people were born with all the brain cells they’d ever have. Researchers debunked that notion in the 1990s, when post-mortem human brain studies revealed neuron growth in brain areas such as the hippocampus2. Some brain functions, scientists now think, might rest on a delicate balance between cell death and growth, as new neurons replace older ones that wither away.
Stressful life events, however, disrupt this delicate balance by affecting rates of hippocampal cell death. People who endure punishing stress show atrophy of the hippocampus3, and brain-imaging surveys have shown that this hippocampal shrinkage is reliably correlated with depressive symptoms4.
Studies of songbirds, which lose brain cells after every breeding season, offer insight into the psychological toll such brain shrinkage can take. Before songbirds mate, neuron counts explode in a brain area that governs motivation, which enables them to produce frequent, mellifluous songs to attract mates. But after the season is over, the birds largely fall silent, and they rapidly lose neurons, says Tracy Larson, a biologist at the University of Virginia in Charlottesville. “The birds lose motivation, but also lose the quality of that singing behaviour.”
When stress alters brain-cell growth in humans, the result might be a similar loss of motivation — in this case, manifesting not as a decline in singing, but as depression. An event such as a major life stressor might lead to cell loss, which then triggers “reactive proliferation”, cell growth that helps brain circuits to recover, Larson says. But in clinical depression, she notes, this compensatory cell growth might not happen. “It could be that the trigger does not mount this reactive proliferation, and that prevents recovery of the circuit.”
Growth and resilience
Scientists have long suspected that some existing depression treatments work, in part, by reversing this brain shrinkage. Researchers at Duke University in Durham, North Carolina, have shown that SSRIs such as fluoxetine don’t just block reuptake of the neurotransmitter serotonin — they also trigger cell growth in the hippocampus and other brain areas5. That growth could help to explain why some people show sustained depression improvement with drug therapy. SSRIs typically take at least two to four weeks to start working. “That’s about how long it takes for a new neuron to migrate from its initial location to where it’s going to incorporate,” says Larson.
More-recent research, such as Belzung’s mouse study last year1, goes further, showing how neurogenesis can directly promote depression resistance. Her team encouraged hippocampal cell growth in some of its 83 mice by blocking a gene that drives cell death in the hippocampus and administering tamoxifen, resulting in more cell growth overall. The other mice received no such treatment.
To induce mild stress in the mice, the researchers tilted the animals’ cages or switched on their lights at night. The results were clear-cut: the untreated mice soon started showing depressive symptoms, whereas the mice with boosted cell growth remained more or less unperturbed. That suggests the growth treatment strengthened the animals’ mental resilience, Belzung says. “If you have more neurogenesis before being stressed, this will protect against depressive-like features.”
Other ways of enhancing brain-cell growth can also stave off depressive symptoms, researchers are finding. Last year, a team in China reported that baicalin, an anti-inflammatory compound, promotes hippocampal neurogenesis in mice that have a depression-like response to mild stress6. The team showed that baicalin boosts production of neural proteins such as Wnt3a, which is involved in a signalling pathway that directs cell growth in the hippocampus. Stressed mice showing depressive behaviour got better when treated with baicalin. Their moods improved about as much as did those of depressed mice on fluoxetine.
Similar findings emerged from a study at Northwestern University in Chicago, Illinois. The researchers encouraged new hippocampal growth in mice using fluoxetine, then chemically activated the newborn neurons that resulted7. This process seemed to relieve depressive symptoms that had already begun. Other studies of SSRI treatment show that these antidepressants activate expression of a protein called brain-derived neurotrophic factor (BDNF), which contributes substantially to cell growth in the hippocampus.
Coaxing out new neurons
Findings such as these could usher in fresh options for depression prevention and treatment. Baicalin’s cell-growth-promoting effects make it a potential candidate for human trials that could test its mettle as an antidepressant. Researchers could also aim to create drugs that act on the Wnt3a protein, BDNF or other targets that control neural growth in the hippocampus. The question for drug developers now, Larson says, is “can we — either in a targeted brain region or in a very controlled manner — create an environment that is supportive of new neurons coming in?”
In addition, Albert says, neurologists could develop magnetic or electrical brain-stimulation protocols that target brain areas such as the cerebral cortex, which receive input from parts of the hippocampus involved in cell growth. The brains of people with or at risk of depression could potentially be scanned to identify areas that have lower-than-normal activity and could benefit from targeted cell stimulation. Such treatment, Albert adds, would probably be geared towards a small group of people — those who have severe depression, or who do not respond to first-line treatments, such as SSRIs.
What complicates treatment prospects, however, is that new cell growth might not be by itself enough to promote resilience or recovery. “Is it just about making new neurons? Or is it about how they’re integrated into the brain?” asks Austin Perlmutter, an independent physician and brain researcher in Portland, Oregon. “I think it’s definitely both.” Belzung concurs, saying that, in her view, neurogenesis seems to be “necessary, but not sufficient” to create depressive resilience.
In other words, after the neurons form, they must become integral parts of the brain’s existing networks, a pruning process in which some new cells get plugged in, while other, unneeded cells die off. “In order to have a neuron incorporate, it needs to have a good solid place to send its projections,” Larson says. “If it doesn’t, it ends up dying.” It’s uncertain how much future neurogenesis treatments would be able to foster such connectivity.
Even if new cell-growth therapies for depression make it through future clinical trials, their precise mode of action could remain a mystery. In part, that’s because techniques that record brain activity in people, such as functional magnetic resonance imaging (fMRI), cannot reliably track individual neurons or the cells they’re connecting with.
Seeing real-time cell changes in the human brain “is very difficult, because of the resolution of the techniques”, Albert says. Future higher-resolution fMRI techniques could help, and post-mortem studies on humans might be able to document more-specific brain changes resulting from cell-growth treatments. However, such studies would take many years to carry out, because they would require comparing brain tissue from people who got these treatments with tissue from people who never did.
Challenges such as these mean it could be years before treatments designed to promote neurogenesis make it to market. But in the meantime, Belzung points out, people at risk of depression can take part in activities known to trigger brain-cell growth. “What you can do is expose people to factors that induce an increase in neurogenesis,” she says. To encourage new neurons to grow and mesh with existing brain networks, Belzung recommends that people with depression exercise or attend social events. Several studies have found that physical activity, in particular, can be a natural way of stimulating neural growth in the hippocampus8.
This line of research suggests there’s a sound biological basis for psychotherapists’ long-standing calls to keep active: exercise encourages brain cells to flourish, in part by driving production of BDNF. That raises the possibility that some people with depression could pursue their own cell-growth-driven recovery through frequent gym visits. “The challenge,” Albert says, “is to find a way — perhaps through activity — to get the person themselves to stimulate [brain cell] connections, like how rehab after a stroke is important to get people to use their limbs again.”
Back-to-basics advice, perhaps, but it comes with more research-backed justification than ever before. To add further detail to the anatomical picture, neuroscientists such as Belzung are planning animal studies that will examine how cell growth in one small part of the brain affects mood and motivation more broadly. “The question is, how is it possible that newborn neurons in the hippocampus can reverse depression?” Belzung says.
Other types of brain cell, she speculates, might play an intermediate part, connecting to new hippocampus cells and sending their input to other parts of the brain. By filling in such knowledge gaps, she aims to clarify just how the right neural growth can prevent — or even outpace — the havoc depression wreaks on the brain.







More News
Author Correction: Stepwise activation of a metabotropic glutamate receptor – Nature
Changing rainforest to plantations shifts tropical food webs
Streamlined skull helps foxes take a nosedive