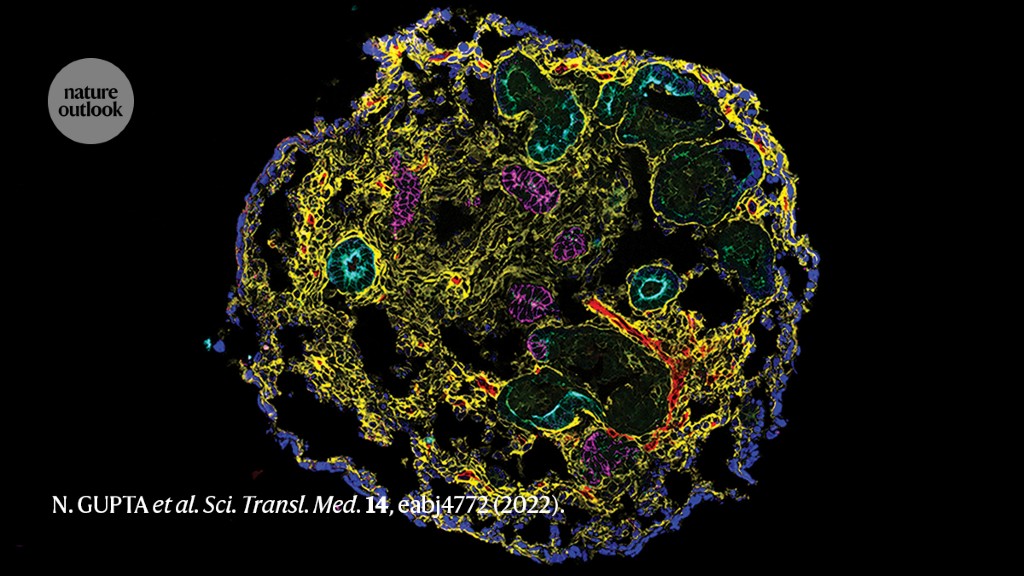
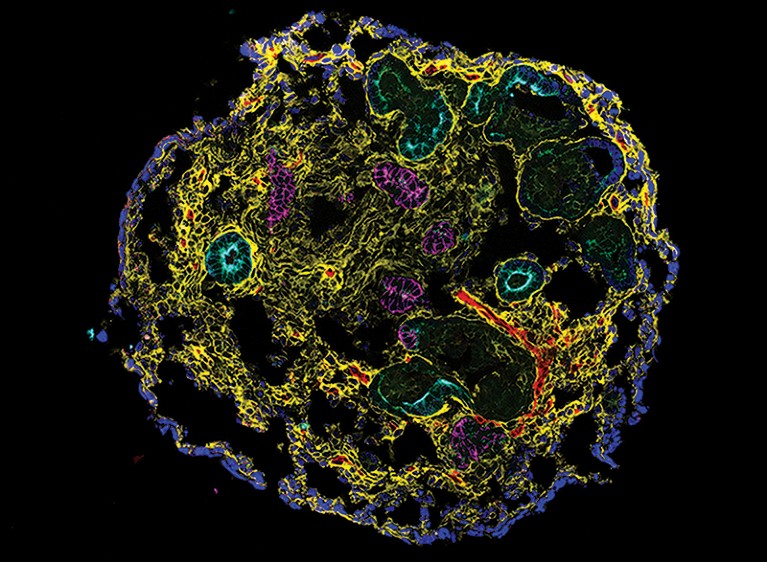
A kidney organoid containing nephron epithelial cells and stromal cells.Credit: N. Gupta et al. Sci. Transl. Med. 14, eabj4772 (2022).
A genome-based risk score for everybody
Current estimates suggest that between one-quarter and a half of a person’s risk of developing chronic kidney disease (CKD) can be attributed to their genes. However, the genetic data on which these estimates are based are dominated by individuals of European ancestry, confounding efforts to develop predictive tests that are broadly applicable across populations. This is particularly problematic because there are known differences in risk factors across racial and ethnic groups. Most notably, people of African ancestry are more likely to carry variants in the APOL1 gene that increase the likelihood of developing CKD. A team led by scientists at Columbia University in New York City has now devised a multi-gene risk score that can identify people at the greatest risk of developing CKD. It is based on the combined effects of multiple disease-linked genomic sequence variants — regardless of a person’s race or ethnicity.
Part of Nature Outlook: Chronic kidney disease
The authors began by identifying gene variants that have a strong statistical association with impaired kidney function, based on a 2019 meta-analysis of genetic data from one million participants. Because this study was skewed towards individuals of European and East Asian ancestry, the authors of the present study also included risk data from a cohort of 7,158 UK Biobank donors of African ancestry. In particular, they focused on the specific risk contributions associated with contributions from variants in the APOL1 gene.
The researchers subsequently tested the performance of the risk score against 15 cohorts from diverse backgrounds. These included six groups of participants of African ancestry, as well as four cohorts of East and South Asian ancestry and two Latinx cohorts. The authors’ score performed consistently across all populations assessed, and individuals who were ranked in the top 2% of risk on the basis of this score were three times as likely to have been diagnosed with CKD. Future iterations of this score could guide preventive care to avert the onset of CKD in people who are at high risk, and potentially guide the screening and selection of organs for kidney donation.
Nature Med. 28, 1412–1420 (2022).
Humanizing kidney-disease research
One challenge in developing effective interventions for CKD is that animal models do not fully reflect human biology, and could fail to predict efficacy and safety in people. A study by researchers at Massachusetts General Hospital in Boston and their colleagues shows how stem-cell-based human organoid models that replicate key structural and functional features of the kidney can provide insight into the underlying mechanisms of CKD that could be clinically relevant.
Starting with induced pluripotent stem cells — adult cells that have been experimentally coaxed into an embryonic-like state — the authors applied a culture system that promoted maturation and self-organization into kidney organoids. These had the distinctive tubular structures expected of kidney tissue surrounded by supporting cells, including fibroblasts and pericytes. This model allowed the authors to investigate the early stages of CKD, when recurrent injury leads to a state in which damage to the kidney tubules is not fully repaired, paving the way for further tissue damage and fibrosis.
After recreating these conditions in organoids, the authors analysed changes in gene expression of the injured tubular cells. Their data highlighted several DNA repair genes that were activated during successful tissue repair, but for which expression sharply dropped off when the tubules were incompletely repaired. The authors then confirmed this shift in expression for a repair enzyme called FANCD2 in biopsy samples.
On the basis of this evidence, they treated their organoid model with a drug that stimulates FANCD2 activity, demonstrating that this treatment could stave off atrophy and fibrosis in damaged tubules. The experiments highlight the promise of human organoid models as a tool for CKD research.
Sci. Transl. Med. 14, eabj4772 (2022).
Immune agents in kidney fibrosis
The formation and accumulation of fibrotic scar tissue is central to CKD progression, and a well-established inflammatory component underlies this process. However, which subsets of immune cells are involved and how these cells interact with kidney tissue is poorly understood.
A team of researchers at the University of Pennsylvania in Philadelphia and their colleagues have addressed this question with an in-depth comparative transcriptomic analysis experiment. Messenger RNA of tens of thousands of cells from healthy mouse kidney tissue was compared with cellular mRNA obtained from the kidneys of an animal model of CKD to identify differences in which genes are activated or inhibited. By using gene expression to classify these various cell types, the authors discovered that fibrotic kidney tissue tends to be highly enriched with basophils. These are a rare subtype of inflammatory cell that has previously been linked to immune diseases of the kidney, but not specifically to CKD.
Subsequent analysis revealed that, in fibrotic kidneys, cells in the proximal tubule — a crucial part of the renal filtration machinery — express a protein called CXCL1 that can actively recruit basophils. These subsequently release an array of inflammation-provoking signalling molecules. Human tissue samples provided further confirmation of this mechanism, with a clearly detectable enrichment of CXCL1 expression and basophil aggregation in fibrotic kidneys. The researchers tested their findings in mice and found that treatments that either selectively eliminate basophils or block the effect of their inflammatory signals can reduce or even prevent fibrosis. The identification of a specific immune-cell subpopulation underlying disease progression should allow the development of treatments that preserve kidney health and function.
Nature Immunol. 23, 947–959 (2022).
A chance to intercept inflammation
Sphingosine 1-phosphate (S1P) is a pro-inflammatory biomolecule that contributes to the formation of fibrotic tissue in the kidney during the progression of CKD. Drugs can be used to block S1P activity by inhibiting its receptor, but these have been shown to perform poorly in people with kidney disease and they can induce serious side effects.
Researchers at the University of Virginia in Charlottesville and their colleagues have now dissected how S1P contributes to kidney fibrosis and identified a mechanism by which they can effectively disrupt this process. They focused specifically on the role of kidney perivascular cells, which line the capillaries of the kidney and communicate closely with the immune system. Earlier work has also strongly indicated that S1P production in these cells is directly involved in the onset of fibrosis.
First, the authors confirmed that inhibiting the production of S1P in these kidney cells quelled the pro-inflammatory response that would normally be elicited in response to kidney injury. Then, they determined that this response is dependent on a protein called Spns2. Perivascular cells use Spns2 to release newly synthesized S1P into the extracellular space, in which it can associate with its receptor and thereby trigger inflammation.
This suggested that there is an opportunity to break the immunological chain of events leading to fibrosis. The authors tested a newly identified small-molecule inhibitor of Spns2 and showed that it could mitigate the inflammatory response in cultured human perivascular cells. They also observed protective effects from this drug in a rodent model of kidney fibrosis, whereas an existing drug that blocks the S1P receptor conferred no such benefit. It remains to be seen whether these therapeutic effects translate to people with CKD, but the initial results suggest that targeting Spns2 could offer a promising avenue for preventing or slowing fibrosis.
Sci. Transl. Med. 14, eabj2681 (2022).
Protecting heart health
Many people with CKD also experience hypertension, a condition that can further exacerbate kidney disease while also worsening cardiovascular health. A clinical trial conducted by researchers at Indiana University in Indianapolis now demonstrates that a drug already used to treat high blood pressure can also confer safe and meaningful protection to people with advanced CKD.
Chlorthalidone is a diuretic drug known to reduce the risk of stroke and heart failure, but there have been some concerns about giving it or related compounds to people with late-stage CKD. To test whether people with this stage of disease might benefit, the authors randomized 160 individuals with severe CKD and high blood pressure to receive 12 weeks of either chlorthalidone or a placebo, alongside a standardized course of other anti-hypertension medications.
More from Nature Outlooks
The treatment arm experienced a notable decrease in blood pressure within four weeks of beginning treatment, relative to the placebo arm. The difference in blood pressure between the two groups persisted throughout the study, and remained statistically significant at 12 weeks, when treatment was discontinued. Beyond this point, the blood pressure of the treatment arm began to rise again. Those taking chlorthalidone also showed other signs of clinical improvement, including weight loss and reduced levels of various blood biomarkers of heart-failure risk. Importantly, those in the treatment arm experienced only a marginal increase in adverse events overall, with fewer serious events requiring hospitalization than in the placebo group.
Although this was a small study with an under-representation of women and people of Asian or Hispanic ancestry, it offers evidence that this drug could protect heart and kidney function in people with CKD. The authors say a phase III trial to demonstrate the efficacy of the drug in a larger cohort is needed.





More News
Author Correction: Stepwise activation of a metabotropic glutamate receptor – Nature
Changing rainforest to plantations shifts tropical food webs
Streamlined skull helps foxes take a nosedive