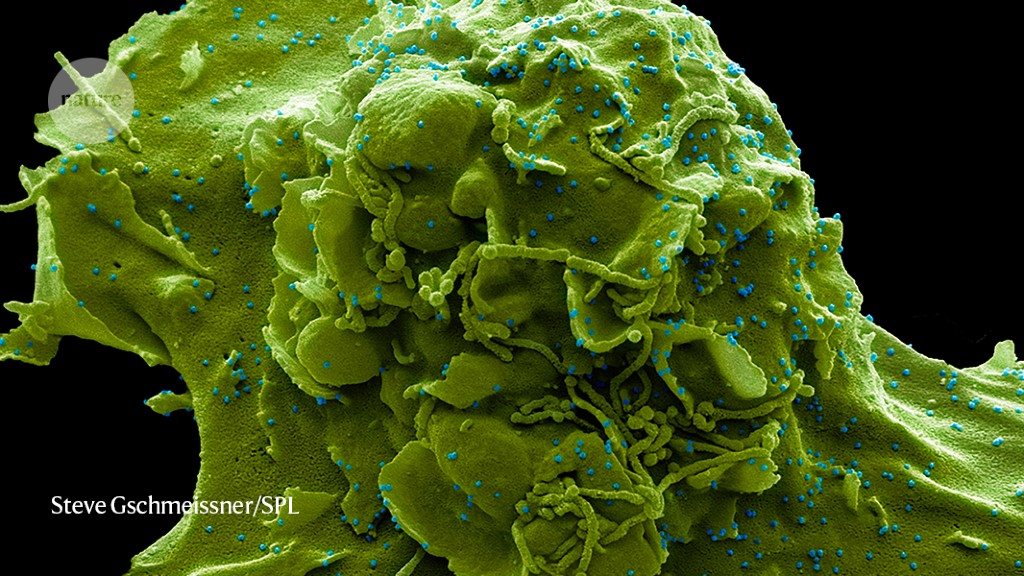
Particles of SARS-CoV-2 (blue; artificially coloured) infect a cell.Credit: Steve Gschmeissner/SPL
COVID-19 can inflict days of misery, even on people who do not develop serious illness. Now, trial data show that an antiviral called ensitrelvir shortens symptoms of mild to moderate COVID-19 by about a day — and is the first drug to make a statistically significant cut in the number of days people test positive for SARS-CoV-2.
The drug’s manufacturer, Shionogi in Osaka, Japan, says the data also show that ensitrelvir has the potential to prevent long COVID. But scientists are sceptical about that claim and critical of the design of the clinical trial. The research was presented at the Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, Washington, on 21 February, and has not yet been peer reviewed.
Two oral antivirals are already widely used to treat COVID-19: Paxlovid (nirmatrelvir/ritonavir) and molnupiravir. Both target people who are at high risk of severe disease. But ensitrelvir was tested on people irrespective of their risk, which could have implications for its use in individuals at low risk. No drug has been conclusively shown to reduce the risk of long COVID, although preliminary evidence hints that Paxlovid might have this effect.
A pill for COVID cough
Organizers of the ensitrelvir trial investigated roughly 1,200 people, with the main goal of determining whether the drug could accelerate recovery. The results showed that participants who took 125-milligram ensitrelvir pills recovered from five specific symptoms — stuffy or runny nose, sore throat, cough, feeling hot or feverish and low energy or tiredness — about 24 hours earlier than did those in the control group.
Participants who took the 125-milligram dose also tested negative for SARS-CoV-2 about 29 hours earlier than did those who took a placebo. According to Shionogi, the study was the first to show a statistically significant reduction in the time to a negative test result.
COVID drug drives viral mutations — and now some want to halt its use
A subset of participants were asked about their COVID-19 symptoms three and six months after trial enrolment as well as during their acute infection period. Those who reported two or more of the same symptoms at least twice in a row over this period were defined as having developed long COVID. Participants who had a relatively high number of symptoms during the illness’s early stages had a 14% risk of developing long COVID if they took the antiviral, compared with a 26% risk for similar participants in the placebo group. This led Shionogi to conclude that participants who received ensitrelvir had a reduced risk of developing long COVID.
Doubts about design
But scientists who were not involved with the study point out that the trial was not specifically intended to investigate the risk of long COVID. That means that the pre-trial research plan didn’t describe any methods for analysing long COVID data.
This means, for example, that it’s unclear whether Shionogi’s definition of long COVID was determined before the trial began, notes physician Eric Topol, director of the Scripps Research Translational Institute in San Diego, California. Because this was an exploratory phase of the study, it’s not possible to make any strong conclusions, he adds.
Simon Portsmouth, head of clinical development at Shionogi in Florham Park, New Jersey, says that the company could not specify the plan for analysing long COVID data ahead of time because long COVID was less clearly defined in the past than now. He says that these results, although not definitive, will shape an ongoing trial evaluating ensitrelvir’s effect on COVID-19 symptoms.
How common is long COVID? Why studies give different answers
Scientists say it’s plausible that antivirals could prevent long COVID. One recent analysis found that people who took Paxlovid had a reduced risk of developing long COVID compared with those who took no antiviral drugs1. The study, published as a preprint, has not yet been peer reviewed. Study co-author Ziyad Al-Aly, chief of research and development at the VA St Louis Health Care System, in Missouri, says the ensitrelvir data make him more optimistic that attacking the virus early during an infection “seems to hold the key to reducing the risk of long COVID.”
Topol agrees that the data that Shionogi have made public support the idea that antivirals protect against long COVID, at least when residual virus is involved in causing prolonged symptoms.
Lingering questions
But there’s no consensus that persistent virus causes long COVID. “It’s entirely possible that the virus has nothing to do with long COVID,” says Edward Mills, a health researcher at McMaster University in Hamilton, Canada. Long COVID might be caused, for example, by the immune response to the virus, he notes.
The optimal study to investigate whether antivirals prevent long COVID would involve selecting only participants whose disease might be caused in part by lingering SARS-CoV-2, says immunologist Danny Altmann at Imperial College London. If scientists don’t separate out such people from those whose symptoms don’t have the same cause, trials could yield “murky answers,” Altmann says.







More News
Judge dismisses superconductivity physicist’s lawsuit against university
Future of Humanity Institute shuts: what’s next for ‘deep future’ research?
Star Formation Shut Down by Multiphase Gas Outflow in a Galaxy at a Redshift of 2.45 – Nature