Menopause is the permanent cessation of menstrual cycles in women following the loss of ovarian function1. It occurs, on average, between 47 and 52 years of age globally2, but around 4% of women undergo it before the age of 45 (early menopause) or even 40 (primary ovarian insufficiency; POI)3. Being able to predict when menopause will occur would give women and their partners greater flexibility in choosing when to have a child. This knowledge, and treatments to delay menopause, might be particularly welcomed by women at high risk of early menopause or POI. Ruth et al.4now report genetic findings that could bring us a step closer to predicting and treating early menopause.
Age at natural menopause (ANM) is determined by the complex interaction of both non-genetic and genetic factors. Non-genetic factors associated with earlier ANM include poor childhood nutrition and smoking, whereas being overweight is associated with later ANM1,2. Genetic factors are thought to account for about 50% of the variation in menopausal timing5. Previous genetic studies6,7 have implicated a role for DNA-damage-response (DDR) mechanisms in the timing of menopause. DDR is a molecular process that is crucial for the error-free replication of cells, including the generation of egg cells in the ovary, and for the repair of DNA damage caused by environmental factors such as cigarette smoke8.
Ruth et al. conducted the largest genetic analysis so far in women whose ANM occurred between the ages of 40 and 60 years, testing millions of common genetic variants from across the genome for an association with ANM. Differences in genetic ancestry could distort results, and the analyses were therefore initially restricted to 200,000 women of European ancestry. Approximately half of this sample was derived from the UK Biobank, a large, population-based study containing extensive clinical, biological and genomic data9. Ruth et al. found 290 independent areas of the genome that contained common genetic variants associated with ANM, a fivefold increase from previous results10. The impact of each variant on ANM varied from very small (3.5 weeks) to considerable (1.5 years).
The authors found that the results from this initial analysis were highly consistent with those from another (non-UK Biobank) data set that they analysed, containing data from about 300,000 women. Most of the implicated variants were also associated with ANM in a third data set that included 100,000 women of East Asian ancestry, although the magnitudes of the variants’ effects varied from those seen in women of European descent. Thus, a genetic test that predicts ANM accurately in one ethnic group might not do so in another.
Altogether, the genetic variants explained around 10–12% of variation in ANM, a reasonable proportion for a trait with complex genetic underpinnings. Using the non-UK Biobank data sets, the authors built a polygenic score (PGS) to predict a woman’s ANM on the basis of the cumulative effect of the common genetic variants that she carries. The authors showed that the PGS was a weak predictor of the ANM of women in the UK Biobank sample, providing slightly, yet significantly, more accurate predictions than did smoking status. Ruth and colleagues noted that women in the top 1% of the PGS score distribution (that is, predicting a lower ANM) had an almost fivefold higher risk of POI than did women with average PGS scores — the same increase in risk as that associated with rare mutations in the FMR1 gene, for which women can currently be tested. The utility and cost-to-benefit ratio of determining the PGS for women in the general population, and in those with a family history of early menopause or POI, remains to be assessed.
Ruth et al. found that many of the variants implicated in their analysis affected genes involved in DDR, including the genes BRCA1 and CHEK2, which have been suggested previously to affect ANM1. Using publicly available gene-expression data from 44 tissue types, the authors found that ANM-associated genes were preferentially expressed in blood-derived stem cells — cell types that have a high turnover and therefore depend heavily on DDR function. The expression of these genes in reproductive tissues such as the ovaries and fallopian tubes, and in human egg and fetal cells, was more variable and requires more-detailed investigation.
Collectively, the genetic data suggested a broader involvement of DDR processes in ANM than previously realized. Earlier research showed that feeding pregnant mice a high-fat, high-sugar diet results in their female offspring having a lowered reproductive potential (reduced ovarian reserve)11. Ruth et al. observed changes in the expression of 2 of 35 assessed DRR-related genes (Dmc1 and Brsk1) in ovarian tissue from the female offspring of mice on this diet, suggesting that maternal diet can affect DNA repair in offspring. However, the impact of changes in expression of the two genes on ovarian ageing was not tested.
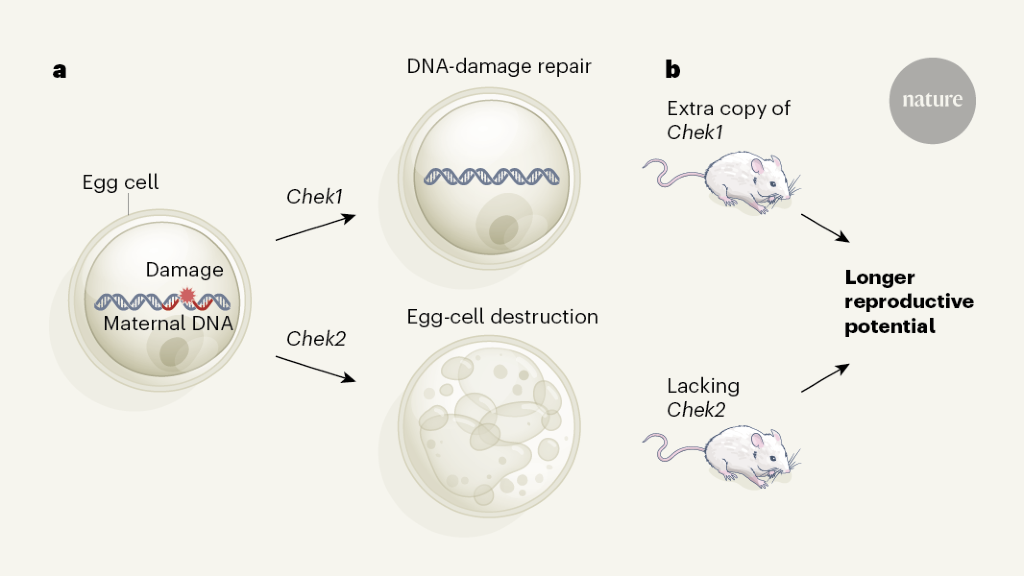
The authors then focused on two DDR genes: CHEK2, which was implicated in their genomic analysis, and CHEK1, which is involved in the same biological (checkpoint kinase) pathway. CHEK1 helps DNA repair, whereas CHEK2 plays a part in the destruction of eggs compromised by DNA damage12 (Fig. 1a).
Inactivating Chek2 in mice reduced ovarian degeneration and, in animals that were around the age of the mouse equivalent of menopause, increased the ovarian response to hormonal stimulation, consistent with these animals having a greater ovarian reserve than that of control mice (Fig. 1b). Fertilization rates in mice lacking Chek2 were unaffected, as were embryonic development and litter size.
Chek1 is needed for embryo development, and its inactivation specifically in egg cells led to female infertility. By contrast, introducing an extra copy of Chek1 resulted in increased ovarian reserve in older mice (Fig. 1b). Thus, limiting the destruction of egg cells or upregulating the DNA-repair process could extend reproductive lifespan in mice.
The authors comment that the mice with an extra copy of Chek1 gave rise to several generations of healthy, fertile offspring, although how the offspring’s health was assessed was unclear. Multi-generational effects of reducing Chek2 expression were not investigated. Any treatments that reduce CHEK2 expression might have adverse effects, however, because CHEK2 is a tumour-suppressor gene, and certain CHEK2 mutations increase the risk of various cancers13. CHEK2 inhibitors are under development for treating cancer, but are unlikely to be suitable for non-cancer-related disorders.
What are the potential health consequences of delaying ANM? Ruth et al. created a statistical instrument to infer how variation in the 290 ANM-associated genomic regions affected various health outcomes in publicly available genomic data sets. This approach revealed that each year of ‘genetically delayed’ ANM increases the risk of hormone-dependent cancers such as endometrial cancer (5%) and oestrogen-receptor-positive breast cancer (3.8%), consistent with epidemiological evidence14. By contrast, genetic variants that delayed ANM were inferred to increase bone density and reduce the risk of fractures, and not to affect the risk of cardiovascular disease or Alzheimer’s disease, lipid levels, body mass or longevity. Notably, the authors’ statistical instrument was based on all known variants influencing later ANM, not just those affecting DDR mechanisms. The effects of manipulations targeting only DDR mechanisms should be investigated.
Many factors determine the reproductive age span, and most — including specific nutritional influences — remain unknown. However, Ruth et al. deliver a considerable advance in our understanding of the genetic and molecular mechanisms that underpin ovarian ageing and ANM. The results will also incentivize further detailed studies into the role of DDR mechanisms in ANM.
The appeal of a future in which women can extend ANM will centre around balancing the risks and benefits, as is the case now for the use of hormone-replacement therapy. For women at risk of early menopause and POI, the benefits might be more likely to outweigh the risks. Although caution should be exercised in translating the findings into genetic tests for early menopause and POI, Ruth and colleagues’ findings pave the way for more-detailed studies that could lead to women being able to predict their menopausal age and to consider options to extend their reproductive age span.
Competing Interests
Financial competing interests: Scientific collaborations in translational research with Bayer AG and Roche Diagnostics, Inc. through the University of Oxford.
Non-financial competing interests: Board member of the World Endometriosis Society and the World Endometriosis Research Foundation. Research Advisory Committee member of Wellbeing of Women UK.





More News
Could bird flu in cows lead to a human outbreak? Slow response worries scientists
US halts funding to controversial virus-hunting group: what researchers think
How high-fat diets feed breast cancer