As we apply insect repellents in an effort to thwart insect-borne diseases, we might stop to wonder how these substances work and why they are not more effective. DEET, the compound most commonly used in insect repellents, is thought to broadly activate insects’ odorant receptor proteins, scrambling the olfactory code the insects use for seeking a host1. But how DEET or natural odorant molecules bind to and affect the activity of insect odorant receptors has not been clear. Writing in Nature, del Mármol et al.2 report the structure of an insect odorant receptor in association with DEET or with the odorant eugenol, thereby providing key insights into how odorants bind to the receptor and how the structure of the activated receptor subsequently changes.
Olfactory systems in different animals have evolved to accomplish highly specialized tasks. A fruitfly homes in on rotting fruit and a mosquito on its host, whereas humans can discriminate between a wide range of food-related scents. Vertebrates and invertebrates alike detect and distinguish between vast numbers of volatile chemicals using a large set of odorant receptors. In many cases, receptor–odorant binding is promiscuous: that is, a single odorant might activate multiple receptors, and each receptor might be activated by multiple odorants. Olfactory neuronal cells each express one type of odorant receptor. Thus, each odorant can activate a different (but sometimes overlapping) set of neurons, creating a combinatorial code to be deciphered by the nervous system3–5.
General principles for odorant coding in fruitflies and humans are remarkably similar. For example, in both species, projections of olfactory neurons expressing the same receptor converge at hub-like structures called glomeruli. However, the receptor proteins in insects and vertebrates could not be more dissimilar. Vertebrate odorant receptors are members of the G-protein-coupled family of receptors6, whereas insect receptors are ion channels that open on binding to an odorant molecule7–10.
This much has been known for decades, but what was not known was how a single odorant receptor could respond to such a large array of structurally diverse molecules. The lock-and-key model of receptor–ligand binding, first espoused by Emil Fischer in 1894, posits that the shapes of a ligand molecule and its binding site in a receptor complement each other exactly11. But this is woefully inadequate for explaining receptor promiscuity12. The structure of an odorant receptor in complex with diverse odorants was needed to shed light on this phenomenon.
To achieve this, del Mármol et al. focused on insect odorant receptors. Most such receptors are assembled from a combination of different subunits (that is, as hetero-multimers), with all receptors containing one particular subunit, Orco, and another, variable subunit that confers ligand specificity13. Previously, single-particle cryo-electron microscopy (cryo-EM) was used to solve the structure of a receptor comprising four Orco subunits14, elucidating the basic architecture of this homo-tetrameric channel. But Orco does not contain a ligand-binding site for the receptor, and so the binding site was not resolved. To solve the structure of a complete insect odorant receptor, while avoiding difficulties associated with resolving the structure of hetero-multimeric proteins, del Mármol et al. focused on an insect odorant receptor that can assemble as a functional homomeric protein in the absence of Orco.
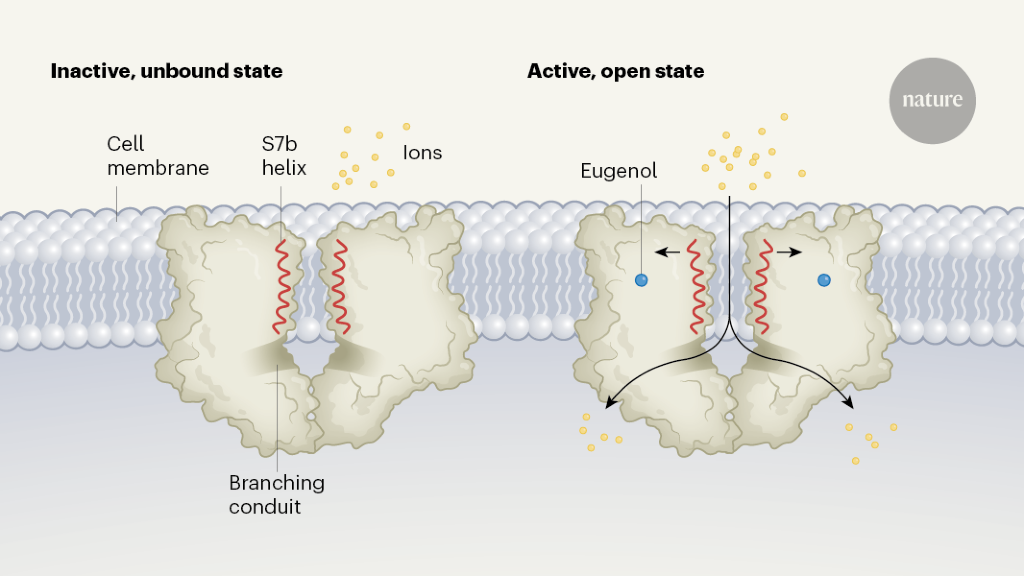
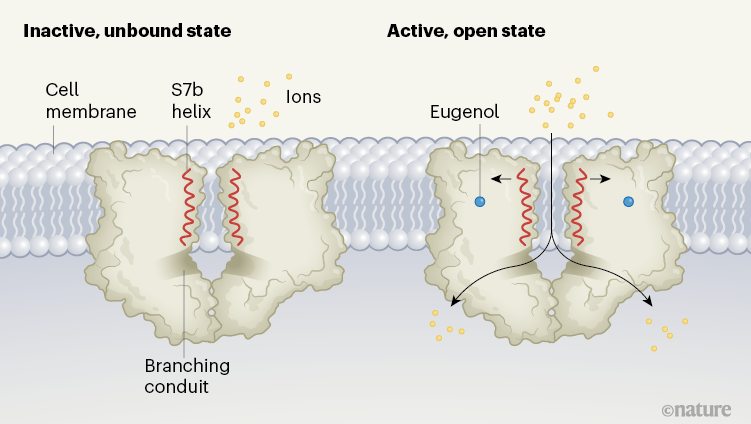
Heteromeric proteins consisting of similar subunits typically arise through a gene duplication that occurs in the process of evolution. Thus, del Mármol et al. reasoned that a functional homomeric odorant receptor would be found in more evolutionarily ancient organisms, and focused on the jumping bristletail Machilis hrabei, a relative of silverfish. In jumping bristletails, the receptor repertoire consists of combinations of just five subunits (MhOR1–5), none of which is directly related to Orco15. Each MhOR5 subunit contains nine α-helices, with six of these spanning the width of the cell membrane (S1–S6), two others (S0 and S7b) partially spanning the membrane and the last (S7a) situated inside the membrane.
The authors tested how channels made from four MhOR5 or four MhOR1 subunits responded to different ligands. They found that MhOR5 channels are broadly tuned, responding to more than 60% of the chemicals they tested (including eugenol and DEET), whereas MhOR1 channels are more selective.
The authors then used cryo-EM to solve the structures of an MhOR5 channel in the unliganded state (known as the apo state), or when bound to DEET or to eugenol (Fig. 1). Overall, the structures they resolved are similar to that of the Orco homomer.
The structures reveal that, when the channel is activated and open, positively charged ions follow a pathway from a large outer vestibule into a single, membrane-spanning conduit that is lined by amino-acid residues from the S7b α-helices of each of the four subunits. On the intracellular side of the receptor, four conduits diverge laterally from the centre, to complete the ions’ pathway into the cell. In the apo state, the narrowest portion of the pathway, measuring 5.3 ångströms in diameter, is found where a valine amino-acid residue in the S7b helix projects into the central lumen, creating a hydrophobic ‘plug’ that restricts ion conduction.
A comparison of apo and ligand-bound states reveals two key architectural features that might explain how the odorant receptor channels, in general, are able to respond to broad arrays of ligands. First, the transition of the channel from a closed to an open state involves only a slight rotation of the S7b helix, which widens the pore and moves a polar residue into the ion-conduction pathway. This movement is likely to require very little energy compared with, for example, the movement of the S4 region during the opening of a voltage-gated ion channel16. Such a low energy requirement for opening might be the key to the ligand promiscuity of the odorant receptor: even the binding of ligands that have low affinity for the receptor can lead to this rearrangement.
Equally important is the nature of the ligand-binding site, which seems to be unusually flexible and able to accommodate ligands of varying chemical composition in multiple orientations. This ligand-binding pocket, located deep within the loose bundle formed by the transmembrane parts of the S2, S3, S4 and S6 helices, is lined by mostly large aromatic and hydrophobic residues that form non-polar interactions with the ligand. This contrasts with the stricter geometric constraints that are imposed by the hydrogen bonds (which are extremely polar) observed in the binding sites of many other types of receptor.
Furthermore, the binding site of the MhOR5 receptor seems to undergo rearrangements to accommodate ligands of different sizes and shapes. Subtle mutations in the pocket change ligand specificity, essentially retuning the receptors. This provides a mechanism by which evolution could have given rise to the large number of receptors with different ligand specificities that are observed in extant insect species.
At long last, 30 years after the discovery of vertebrate odorant receptors6, we have the structure of an odorant receptor in complex with its ligand. But, much as this advance answers age-old questions, it also raises new ones. For example, the structures of neither the apo nor the ligand-bound receptors show a pathway for odorants to access the ligand-binding pocket. One possibility is that the channel ‘breathes’, transiently opening a pathway through which odorants can get to this site and thereby lock the channel in an open conformation. Defining the odorant-access pathway through studies of the structure and function of the receptor might provide further insights into receptor specificity.
This and previous studies (for example, ref. 9) also raise the question of how a system in which receptors are active in the absence of a ligand (albeit at a low level) can distinguish between the signal from ligand-bound receptors and the noise of active, but ligand-free, receptors. One possibility is that the olfactory system can tolerate a certain amount of noise, because it can continue to collect and integrate new information over time: for example, as an insect nears an odour source, it might use new, higher-quality olfactory information to correct its course. Another possibility is that the organization of the olfactory system into glomeruli that pool information from many neurons effectively filters out activity of single errant neurons. Analyses of the processing of odorant signals in glomeruli and by the central nervous system might be needed to answer these questions.
Finally, one wonders whether these findings can be generalized to describe vertebrate G-protein-coupled odorant receptors. Like their insect counterparts, vertebrate odorant receptors bind to odorants mainly through non-polar and weakly polar interactions17. Might they also be endowed with a flexible ligand-binding pocket and low energy barrier for engaging downstream signalling, which together enable them to respond to various relatively low-affinity ligands? Undoubtedly, the work of del Mármol et al. and future studies will lead to the development of designer ligands for odorant receptors, and perhaps even, someday, to a substitute for DEET.



More News
Chance of heatwaves in India rising with climate change
A site-resolved two-dimensional quantum simulator with hundreds of trapped ions – Nature
How AI could improve robotics, the cockroach’s origins, and promethium spills its secrets