Preliminary results from a landmark clinical trial suggest that CRISPR–Cas9 gene-editing can be deployed directly into the body to treat disease.
The study is the first to show that the technique can be safe and effective if the CRISPR–Cas9 components — in this case targeting a protein that is made mainly in the liver — are infused into the bloodstream. In the trial, six people with a rare and fatal condition called transthyretin amyloidosis received a single treatment with the gene-editing therapy. All experienced a drop in the level of a misshapen protein associated with the disease. Those who received the higher of two doses tested saw levels of the protein, called TTR, decline by an average of 87%.
The treatment was developed by Intellia Therapeutics of Cambridge, Massachusetts and Regeneron of Tarrytown, New York. They published the trial results in The New England Journal of Medicine1 and presented them at an online meeting of the Peripheral Nerve Society on 26 June.
Previous results from CRISPR–Cas9 clinical trials have suggested that the technique can be used in cells that have been removed from the body. The cells are edited and then reinfused back into study participants. But to be able to edit genes directly in the body would open the door to treating a wider range of diseases.
“It’s an important moment for the field,” says Daniel Anderson, a biomedical engineer at the Massachusetts Institute of Technology in Cambridge. “It’s a whole new era of medicine.”
Treating the untreatable
Transthyretin amyloidosis is caused when molecules of TTR protein fold into the wrong shape and clump together, forming fibres that interfere with organ function. The disease principally attacks the heart and nerves. A hereditary form of the condition affects about 50,000 people worldwide; more than 100 mutations in the TTR gene have been linked to transthyretin amyloidosis.
The condition can cause heart disease, pain and death, and until recently there was little that doctors could do to treat it. “It’s an absolutely awful disease,” says Julian Gillmore, a nephrologist at the Royal Free Hospital in London who has treated people with various forms of amyloidosis for about 25 years. “Up until a few years ago, I just watched them get worse and die.”
But transthyretin amyloidosis has become a prime target for new medical technologies that aim to disable disease-causing genes. It is definitively linked to production of a particular protein — shut down production of that protein, and disease symptoms stop progressing and in some cases reverse.
In 2018, the US Food and Drug Administration approved two drugs to treat transthyretin amyloidosis, both of which reduce the production of TTR by targeting the messenger RNA that encodes it. But although these drugs reduce TTR by about 80% and prolong survival, they must be taken routinely to keep TTR levels down, and do not always halt disease progression.
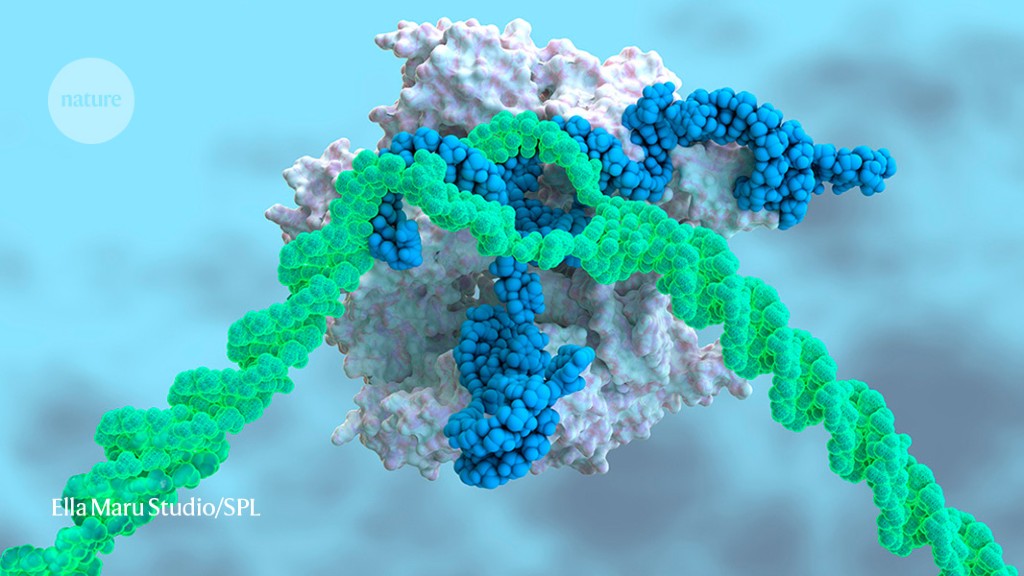
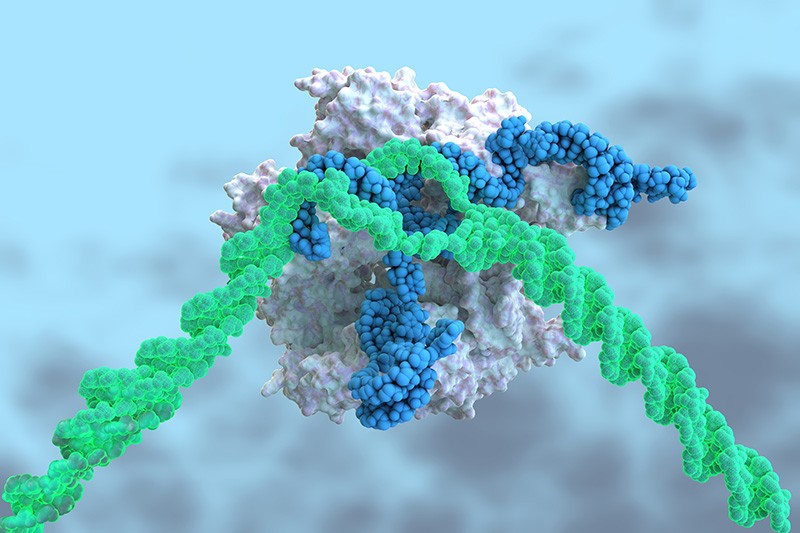
Most TTR protein is produced in the liver, an organ that readily soaks up medicines from the blood. That feature is key: although CRISPR–Cas9 has been hailed as a revolutionary way to treat diseases, it has limitations. The technique requires a DNA-cutting enzyme, called Cas9, and a piece of RNA, called a guide RNA, to direct it to the proper place in the genome. These must be packaged in a way that shields them from degradation, and must be delivered to the site in the body where they are needed.
Intellia encased RNA molecules coding the guide RNA and the Cas9 protein in nanoparticles made of biomolecules called lipids, which can be taken up by the liver. The guide RNA sends Cas9 to snip the TTR gene; the cell’s DNA-repair processes then mend the break, but often make mistakes that can disable the gene and halt production of the TTR protein.
A month after treatment, TTR production was reduced in all participants. In one person who received the higher of the two tested doses, it fell by 96% — a result that Gillmore, who was an investigator on the trial, finds particularly exciting. Current treatments can reduce TTR by as much as 80%. But he says that a therapy that knocks TTR production down further would increase the chance that his patients’ symptoms would stop getting worse — and might even lead to improvement.
“When you get to numbers like 96%, that’s where you start to give the body a chance to clean up what has been deposited,” says John Leonard, president of Intellia.
Bone marrow and beyond
Researchers will anxiously await data from more participants, says Anderson, and will want to know how all the participants fare over a longer period of time. But the current results are enough to fuel hope that CRISPR–Cas9 will one day be able to help treat not only transthyretin amyloidosis, but other conditions, too.
Intellia has also shown that it can deliver CRISPR–Cas9 components to cells in the bone marrow in mice. Leonard says the company is keen to develop a method of treating sickle-cell anaemia that doesn’t require the arduous and risky bone marrow transplant used in ongoing gene-editing trials for the illness.
Another company, Editas Medicine in Cambridge, Massachusetts, has encoded CRISPR–Cas9 components into a disabled virus. Editas is testing this in people with a hereditary disorder that causes blindness, but the virus must be injected directly into the eye, where the gene editing needs to take place.
Techniques for delivering CRISPR–Cas9 components to various parts of the body are advancing rapidly, says Anderson. “The list keeps growing,” he says. “I’m optimistic that we’re going to see much broader application of genome editing.”




More News
Could bird flu in cows lead to a human outbreak? Slow response worries scientists
US halts funding to controversial virus-hunting group: what researchers think
How high-fat diets feed breast cancer