The human body consists of trillions of cells that perform innumerable tasks as members of different organ systems. They are all descendants of the fertilized egg, which divides again and again to generate large numbers of progeny during embryonic development. Later in life, cells continue dividing to compensate for cell death and to ensure consistent tissue function. The ancestral relationships between the body’s cells can inform us about their division and migration histories. Writing in Nature, Park et al.1, Coorens et al.2, Li et al.3 and Moore et al.4 provide insights into human embryonic development and tissue maintenance by uncovering the lineage relationships between cells that reside in different parts of the body.
The common principle uniting the four studies is that they use mutations in the genome as markers for tracing lineage. Throughout their lives, cells continually acquire random mutations that are passed on to all their descenwdants as permanent tags. A cell’s mutation profile therefore represents a fingerprint that encodes its ancestry back to the fertilized egg. By sequencing the genomes of cells from different parts of the body, ancestral relationships can be determined and a cellular ‘family tree’ can be constructed, enabling a retrospective view of the cells’ provenance and past behaviour.
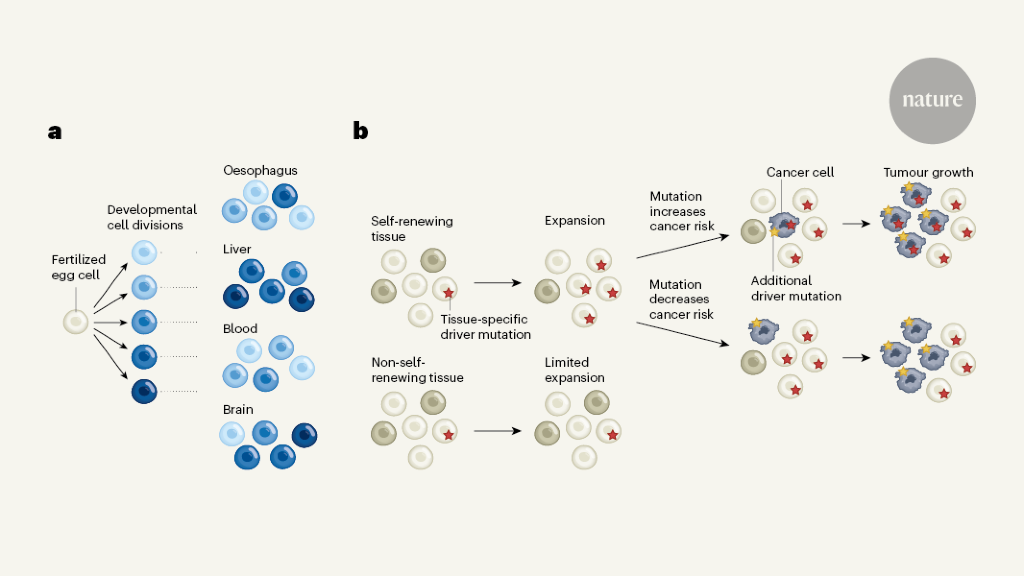
The four studies showcase several fascinating applications of this principle. Park et al.1 and Coorens et al.2 shed light on the earliest stages of human embryonic development. They inferred the mutation profiles of individual cells from tissues collected from recently deceased adults at autopsy and constructed cell-lineage trees that visualize how the cells relate to each other. The branching points of such trees represent cells that existed in the past and can resolve the first embryonic cell generations in great detail (Fig. 1a).
Consistent with previous work5–7, Park et al. and Coorens et al. observed that the first two discernible lineages (which could well be the two cells that arise from the first division of the fertilized egg) contribute variably to the body’s tissues. This finding highlights the stochastic nature of cell-fate decisions that take place during early development. Moreover, of the first eight cells to arise, only approximately three give rise to the embryo, whereas the rest separate from these cells to form other tissues outside the embryo, such as the placenta.
Both Park et al. and Coorens et al. also observed that the mutation rate is relatively high, approximately 2.4 mutations per cell per generation, during the first few embryonic divisions. It then drops considerably, at about the same time that cells activate more-mature DNA-repair mechanisms.
Cells in the early embryo mix extensively, so that physical proximity does not always suggest relatedness. For example, Park et al. observed that adjacent connective-tissue cells can derive from lineages that segregated with the first embryonic division. However, some lineages that arise after the third generation become enriched in organs that derive from a layer of cells in the early embryo called the ectoderm, and mutations that arise after approximately six to nine generations can become enriched in specific organs. Resolution of developmental events beyond the first 15 cell generations or so will require the analysis of more cells from more tissues, but the current analyses already provide a tantalizing taste of what the future holds.
Li et al. and Moore et al. demonstrated that mutations can also reveal important insights into the biology and evolution of tissues in later life. To achieve this, the authors sequenced the genomes of small groups of cells dissected from different organs, and examined the abundance and diversity of mutations in them.
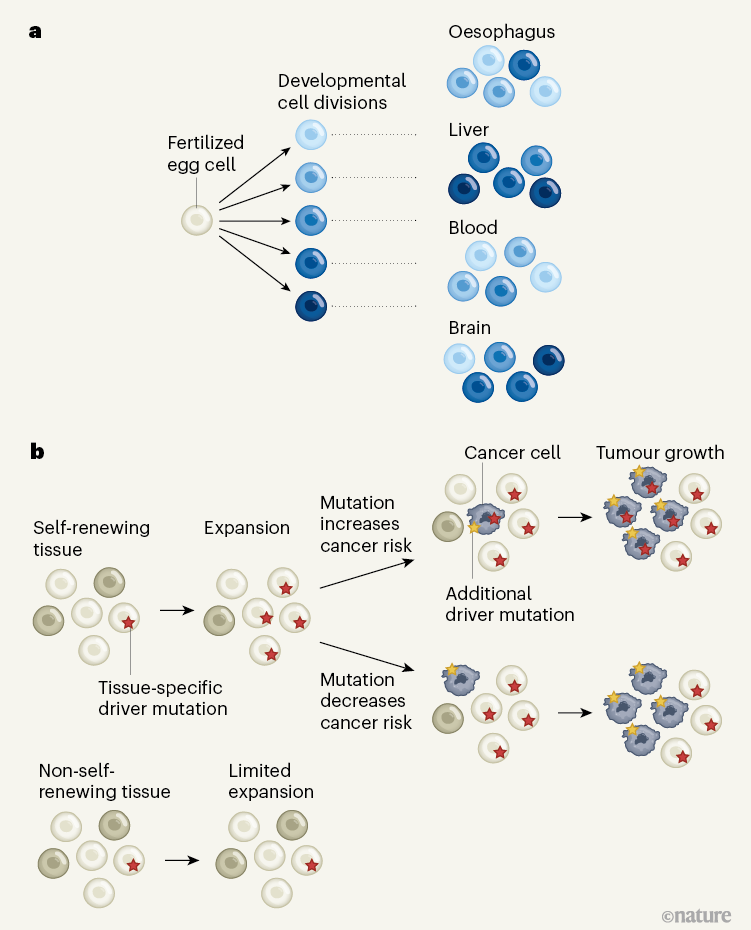
Moore et al. observed that many known tissue structures that are visible under the microscope, such as the folds of the inner lining of the small and large intestines, consist of genetically similar cells that all descended from a recent common ancestor — a tissue-resident stem cell. By contrast, tissues that do not self-renew, such as muscle and brain, typically contain genetically different cells with no recent shared ancestry.
Furthermore, by examining the mutations that accumulate in different tissues, it is possible to pinpoint and quantify the molecular mechanisms that introduce mutations into the genome in different cellular environments. For example, both Li et al. and Moore et al. showed that, in contrast to other tissues, a high fraction of the total mutations found in gastrointestinal tract cells are attributable to cell division, whereas liver cells are vulnerable to mutations resulting from exposure to toxins originating outside the body. Moore et al. observed that precursors of sperm cells in the testes acquire mutations at an unusually low rate, potentially hinting at special DNA-repair mechanisms that protect the genetic material passed on to the next generation.
In addition to the ubiquitous neutral mutations, some cells acquire ‘driver’ mutations that affect their behaviour and that might cause them to contribute to a disproportionately large fraction of a tissue’s cell population (Fig. 1b). Li et al. found that different genes cause such abnormal cell behaviour in different tissues, and, to some extent, in different individuals. In some tissues, populations carrying a particular driver mutation are constrained in their expansion by microanatomical structures in the tissue, and therefore typically remain small. In tissues with a ‘flatter’ anatomy, however, a mutant population can expand to encompass large areas, without obviously affecting tissue function adversely.
Together, the four studies provide an impressive demonstration of the power of modern genetics to decode the cellular dynamics that unfold in our bodies over time. Scaled-up versions of these experimental designs will provide insights into how organs are formed and, crucially, deepen our understanding of diseases caused by harmful mutations that sometimes arise during embryonic development.
From the point of view of human health, tissue evolution during later life is probably an even more pressing topic than embryonic development. Already, our conceptual understanding of how cancer develops has been shifted profoundly by the recognition that healthy tissues can contain mutations that were previously thought to be relatively specific to cancers8. It is becoming clear that some of these alterations might not drive cancer at all, but might simply be inherited from normal cells by cancer cells. Some mutations that spread throughout normal tissues might even protect against cancer9,10.
Li et al. report that a notable fraction of tissue samples across different sites, such as the oesophagus and the rectum, contained three or more mutations thought to drive cancer — although it is not clear whether these driver mutations were present together in the same cell. This is consistent with previous work demonstrating the presence of up to three driver mutations in normal airway cells from people who smoke11. Three driver mutations is uncomfortably close to the average four or five that are found in cancers12, particularly given the limited sampling of normal tissue so far. Indeed, if cells with three driver mutations can easily be found in a small tissue sample, cells with four or five drivers probably exist in that tissue as well — without necessarily giving rise to cancer.
These new insights invite us to reconsider how we genetically define cancer. If having multiple driver mutations does not make a cancer, what does? Is a particular, tissue-specific combination of mutations required? Or is the presence of such mutations required in addition to permissive environmental conditions? Chromosomal abnormalities have often been cited as being specific to cancer cells, but both Li et al. and Park et al. report that normal cells in some tissues contain chromosomal changes as well.
It is likely that full clarification will be possible only with the generation of a ‘normal-tissue genome atlas’, in which the mutational composition of different tissues is carefully mapped across many individuals as a function of age, medical history and lifestyle. Only then can we hope to answer the foundational question about the genetic definition of cancer with some rigour.
Competing Interests
The author declares no competing interests.



More News
Could bird flu in cows lead to a human outbreak? Slow response worries scientists
US halts funding to controversial virus-hunting group: what researchers think
How high-fat diets feed breast cancer