One day soon, a person might be able to find cavities long before they appear on a dental X-ray, and early enough that the decay can be stopped in its tracks. All it would take is an instrument similar to an electric toothbrush that is fitted with a camera and an LED instead of a brush. You would rinse with a special mouthwash, run the instrument over your teeth, then look at your smartphone to see which areas show signs of acid eating away at the tooth enamel.
Share the images with your dentist, and she or he could prescribe a highly concentrated fluoride solution to be carefully applied to the danger spots. Then you would check again a few weeks later to see whether the area had remineralized, and adjust the treatment if necessary.
That is the vision of Eric Seibel, director of the University of Washington’s Human Photonics Laboratory in Seattle. He has filed a patent on this technique and is trying to find a medical-device company interested in building such an instrument.
Seibel is one of several researchers developing optical-imaging techniques for dentistry, using light instead of X-rays to examine people’s teeth for cavities and cracks. Light in the near-infrared spectrum can penetrate the outer enamel layer, the underlying dentin, the pulp at the centre and even the gum tissue.
Part of the motivation is to reduce patients’ exposure to ionizing radiation, even though the risk from dental X-rays is extremely low. The UK National Health Service says that the lifetime increase in risk of a fatal cancer from each standard dental X-ray is one in a few million. However, for children, alternatives to X-rays would be especially welcome, both because the radiation dose is larger relative to their body size and because children’s teeth change rapidly enough that more-frequent imaging could be useful. Dentists and dental assistants, who might be near X-rays on a daily basis and thus face a higher risk of exposure than most people, could also benefit.
But the main reason for the interest in optical imaging is that it can visualize problems that X-rays can’t. “The problem with an X-ray isn’t just that it uses ionizing radiation — it’s also not very sensitive for certain types of lesion,” says Daniel Fried, a researcher in the School of Dentistry at the University of California, San Francisco. Alternative techniques could enable dentists to spot cracks and the beginnings of cavities that are too small to show up on X-rays.
Early warnings
Teeth are well suited to optical imaging because of their make-up, says John Girkin, a physicist who directs the Biophysical Sciences Institute at Durham University, UK. “The enamel is basically an optical fibre, so it guides light down it,” he says. “What happens in early disease is, this optical fibre gets damaged, and instead of the light going through the tooth, it gets scattered back. So you end up with these white spots.”
These white lesions can be visible to the naked eye, but are often hard to spot against the white enamel. They are early signs of tooth decay, before a large cavity has developed. When they are small they can be treated with fluoride, which causes the weak area in the enamel to remineralize. “The tooth, if you can detect the problem early enough, is capable of healing itself,” Girkin says. “But the problem is when something appears on an X-ray, it’s gone beyond the point at which it will heal itself.”
Girkin’s background is in optics — his PhD thesis was on laser spectroscopy of atomic hydrogen — and he has long had an interest in developing medical instruments. That led him to think about which optical technologies might take advantage of the light-transmitting characteristics of tooth enamel. He initially tried multiphoton microscopy, firing short pulses of laser light into a tooth to excite fluorescence and then looking for areas where the tooth didn’t fluoresce as much, which would indicate the presence of damaged tissue. It worked nicely on extracted teeth in a holder on an optical bench, he says, but seemed too tricky to use in a clinical setting.
In trying to avoid the use of complex equipment and fluorescence measurements, Girkin came up with the idea of using a readily available infrared light source and a commercial camera capable of detecting the right wavelength. He shone the laser beam from a CD player through a tooth and into a webcam. “Lo and behold, you can take a transmission image through a tooth with very high quality,” he says.
His next step was to develop this method into a 3D image of a tooth. To obtain images from different depths in the tooth, he projects a pattern of lines and spaces into the structure. Near-infrared light with a wavelength of 850 nanometres penetrates the tooth, but the photons don’t all go straight through. Instead they are scattered, blurring the pattern. The amount of blur increases as the photons penetrate further into the tooth — providing a way to measure depth. In places in which lesions are present, the effect breaks down, light transmission drops and the lesion shows up as a dark spot in the image.
Girkin moves the pattern and repeats the process until he has imaged the entire tooth. The result is similar to computed tomography (CT) imaging, in which a 3D image is built up layer by layer. The image, which can be colourized to make problems more visible, shows the depth and size of the decay, making it easier for dentists to decide on the best treatment. Most optical 3D scanners that are already in use by dentists image only the surfaces of teeth; if the aim is to construct a replacement tooth, that provides enough information.
Seeing lesions that an X-ray would miss is not the only advantage of Girkin’s technique. Because it uses near-infrared light, which is harmless to tissue, dentists can perform this type of imaging as often as needed. They can prescribe a fluoride treatment for the affected tooth, then check a few months later to see whether it’s working. Girkin has founded a company, NirVisio, to develop the technology into a clinical instrument and is seeking funding to run clinical trials.
Even longer wavelengths
Fried, however, is interested in going even further into the infrared spectrum. “When you go all the way out to around 1,300 nm, that’s when the enamel is most transparent,” he says. “It’s almost like glass. You can see right through it.”
Using light with a longer wavelength, Fried says, produces clearer images than the wavelength that Girkin uses (as Girkin also notes), and is much better for spotting decay than X-rays. Stains that might hide tooth decay become invisible under that light. Fried can also shine light into the gum and see it come out through the top of the tooth (known as the occlusal surface). This allows him to spot cavities beginning to form on the top of the tooth — a useful advance, because a tooth’s occlusal surface is typically studded with pits and it can be difficult to use an X-ray to distinguish an early cavity from a natural indentation.
Although shining light through teeth to find problems has proved valuable, Fried says looking at the light reflected off the tooth can work even better in some cases. This technique involves shining a light down on top of the tooth and looking at what bounces back. Demineralized lesions — which are more porous than the surrounding enamel — scatter more light, so they appear brighter. At the right wavelengths, the difference in scattering can be measured in orders of magnitude, providing a high contrast between healthy and diseased tissue. Fried is looking at wavelengths between about 1,450 nm and 1,950 nm for that application; one study that he and his colleagues published earlier this year showed that 1,950 nm seemed to be the best wavelength for visualizing lesions clearly1. Using multiple techniques at different wavelengths in the same examination has the advantage of potentially weeding out false positives that might result from using this more sensitive imaging method.
The downside is that the detectors for these wavelengths are made of indium gallium arsenide — rare materials that are therefore expensive. The 850-nm light that other researchers use can be detected with the inexpensive cameras typically built into a smartphone.
One technique that is often mentioned in the dental-imaging literature is called optical coherence tomography (OCT). Over the past 30 years, OCT has found uses in a variety of medical imaging technologies, from identifying cancer to examining retinas and coronary arteries, but it has yet to be used for dentistry. OCT is often compared to ultrasound, because it also penetrates tissue to build up a high-resolution 3D image. It uses beams of near-infrared light to probe deep into the tooth, and creates images with a resolution measured in micrometres. It can image severe decay that has spread from the enamel to the underlying dentin, even when the decay doesn’t show up on an X-ray. However, this technology scans only a small area at once, and many experts consider it too slow for dental uses, as well as too expensive.
But Fried says scanning speeds have increased considerably and thinks that costs could come down if a manufacturer of medical equipment devoted efforts to building OCT machines more efficiently. He attributes the lack of such scanners more to business questions, such as whether dentists will consider the machines worth the cost, than to any technical issue, and expects they will eventually enter the field of dentistry, “I’m pretty confident they will become available,” he says.
Go with the glow
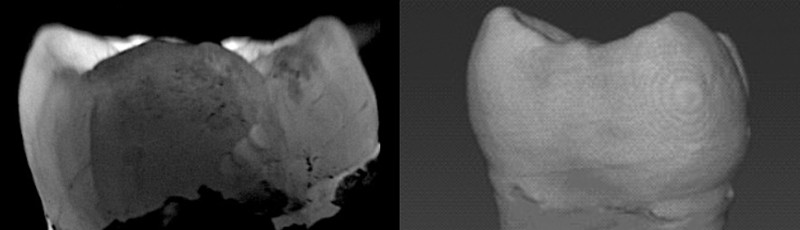
One dental problem that is nearly impossible to detect early — either using X-rays or by inspections with the naked eye — are tiny cracks in the enamel, which can make teeth sensitive and provide an entry point for bacteria. By the time such cracks become visible using existing imaging techniques, they’re large enough to require a root-canal procedure or tooth extraction. To identify these problems earlier, Jian Xu, an electrical engineer who studies biomedical imaging at Louisiana State University in Baton Rouge, turned to fluorescence. By using a fluorescent dye, Xu and his colleagues showed in a 2019 study that they could find cracks that were only 3 µm wide2. X-rays cannot detect those cracks until they have grown to at least 15 µm, he says.
The technique relies on indocyanine green, a dye that has been approved for various types of medical imaging since the late 1950s. Although it is usually injected for those diagnostic methods, Xu says that, for dental imaging, the dye could be put in a mouthwash. The enamel absorbs the dye and fluoresces when it is hit with a laser beam at near-infrared wavelengths. The crack appears as a shadow in the image. Xu has performed his studies on extracted teeth and is developing protocols for a clinical trial, which he expects to begin within the next two to three years.
The acid-measurement technique being developed by Seibel at the University of Washington also relies on fluorescence. Cavities develop when bacteria on the teeth digest sugar and excrete acid, which eats away at the enamel. So Seibel focuses on finding areas on the teeth that are more acidic.
Like Xu, Seibel uses a fluorescent dye called fluorescein that is already approved by the US Food and Drug Administration. This dye can be excited with violet light at 420 nm and has fluorescent peaks at two different wavelengths, which can be used to give a more accurate reading, and the strength of the fluorescence depends on the pH. If the pH is acidic, indicating the breakdown of enamel, restorative action can be taken. “You have a few months to apply topical fluoride and rebuild it,” Seibel explains. However, topical fluoride does have to be applied carefully to avoid overdoses, which can discolour teeth and cause other side effects.
None of these techniques will completely replace X-rays, the researchers say. “X-rays are used for other purposes as well, not just cavity detection — for example, looking at the bone,” Fried notes. “However, I do see the potential for reducing the number of screenings and, I think most importantly, you could do things that you can’t do with radiographs.”
But optical imaging techniques that can catch problems early might render some X-rays unnecessary. And if techniques that use infrared LEDs or fluorescent mouthwashes make their way into daily dental practice, they could light the way to better oral health.






More News
How artificial intelligence is helping Ghana plan for a renewable energy future
Editorial Expression of Concern: Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase – Nature
Quantum control of a cat qubit with bit-flip times exceeding ten seconds – Nature