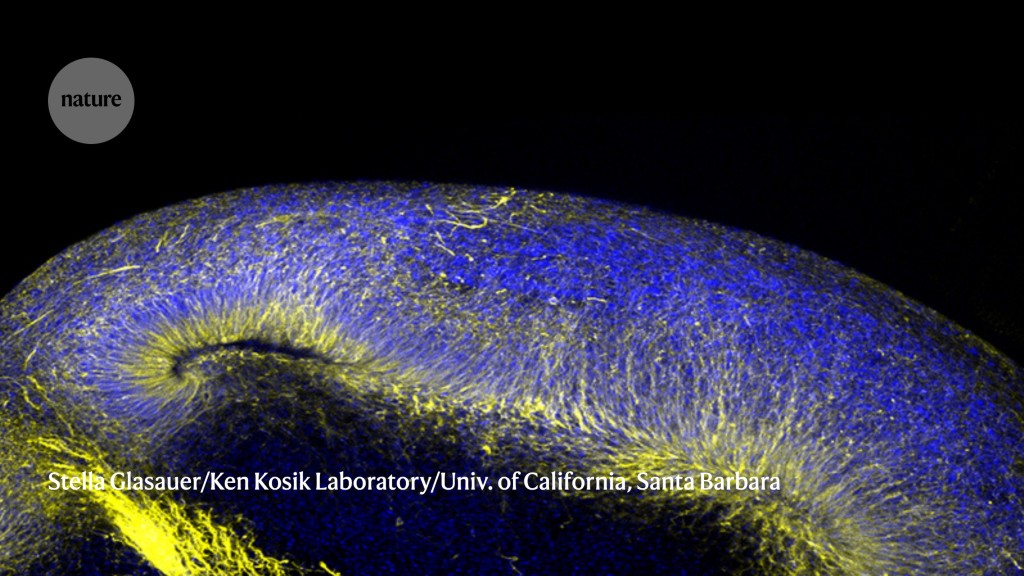
Miniaturized versions of human brain tissue, these organoids are a powerful tool for studying neurodegenerative disorders.Credit: Stella Glasauer/Ken Kosik Laboratory/Univ. of California, Santa Barbara
People born with chordoma, a rare cancer affecting the spine, have few treatment options. Many common cancers can be tackled with a plethora of specialized and targeted treatments, but chordoma is typically addressed with surgery and chemotherapy. The median survival from diagnosis is roughly seven years.
Researchers face many constraints in trying to develop therapies for this cancer. It grows very slowly, making it difficult to culture, and it is also exceedingly rare, affecting fewer than one in a million people worldwide. But cancer biologist Alice Soragni of the University of California, Los Angeles, has devised a promising solution1: cultivating biopsies into self-organizing, 3D ‘organoids’ that retain the characteristics of tumour tissue, and then using them to screen against a battery of drug candidates.
Soragni thinks this could be a game-changer not just for chordoma, but for myriad other unusual tumours. “Depending on the definition, rare cancers can encompass up to a quarter of all diagnoses,” she says. But this is only one aspect of the excitement surrounding organoids, and these miniaturized versions of human tissues are now proving their mettle as a powerful tool for studying, and potentially treating, diverse diseases affecting almost any organ.
Just act natural
Many biomedical studies are done in suboptimal models that do not reflect the reality of the body. These include cell lines with cancer genes used to promote continuous cell division, cultivated on a 2D plastic surface. Such cell lines are easy to grow, but hard to trust. “Those cell lines are basically cancer cells, and are very different from normal cells in our human body,” says Jie Zhou, a virologist at the University of Hong Kong. Animal models, including genetically modified rodents and ‘xenograft’ models in which mice are implanted with tumour tissue, can offer better outcomes, but these are expensive and labour-intensive to generate. Some major diseases, such as Alzheimer’s, lack good models entirely.
Nature Index 2022 Biomedical sciences
Organoids can offer an excellent solution for many of these problems. By cultivating human-donor-derived cells in the right conditions, researchers have shown that they can coax these cells to self-organize into 3D assemblies that capture key features of the structure and function of a diverse collection of healthy and diseased tissues. This can greatly reduce the need for animal testing, while yielding much more naturalistic models of the body. For example, Zhou’s group has developed an array of sophisticated organoid systems that can recreate the entire human respiratory tract — from nostrils to alveoli — for studying the pathology of respiratory viruses.
Much of her lab’s recent work has revolved around characterizing the infectious behaviour of different variants of SARS-CoV-2, and access to a more realistic model of the human airway has allowed her to make valuable insights. For example2, whereas many studies of the Omicron variant have focused on its ability to elude the host immune response as a key mechanism underlying its rapid spread, “we are showing that Omicron also replicates better than Delta and the ancestral strain” of SARS-CoV-2, Zhou says. She adds that conventional cell lines do not capture this behaviour. Her group is currently applying this approach to the now-dominant BA.5 strain of Omicron, and is also looking into the use of airway organoids as a better tool for studying the effectiveness of different SARS-CoV-2 antibodies.
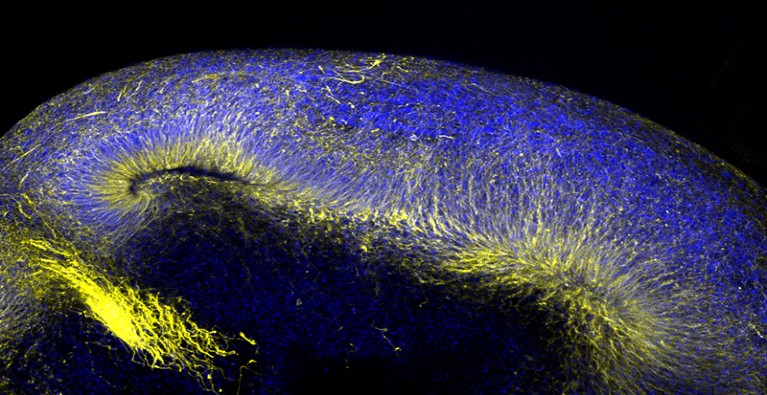
Even complex organs such as the brain can be reconstructed in this fashion, and neurobiologist Kenneth Kosik of the University of California, Santa Barbara, is working with organoids that capture some surprisingly sophisticated features of the human cerebral cortex3. “You get regular spiking, just like you would in the brain,” he says, referring to the electrical signatures that reveal synaptic activity, adding that his team has even observed signs of higher-order neural circuit formation in their system.
These systems offer a rare opportunity to directly view how disease-related genetic mutations derail brain function, and Kosik is interested in getting a better understanding of neurodegenerative disorders. Because brain tissue is hard to obtain, his organoids are generated from patient-derived induced pluripotent stem (iPS) cells, which are mature cells that have been biochemically coaxed into re-entering an embryonic state and so can yield any cell type in the body. The resulting organoids resemble the embryonic cerebral cortex more closely than adult tissue, but can capture crucial early events in disease development.
In a study published earlier this year, Kosik’s group used iPS cells with mutations in the tau protein that commonly gives rise to dementia to generate cerebral organoids4. The resulting models exhibited striking abnormalities in cholesterol processing that could ultimately impede neuronal function and survival. “It means that these cells just don’t like having this mutation around,” he says. His lab is also investigating similar models as a tool for studying rare neurodevelopmental disorders that typically manifest at birth or early childhood.
Organoids can provide a highly personalized tool for guiding medical treatment. This is particularly important for cancer, in which a malignancy can arise primarily from a particular ‘driver’ mutation, but with assistance from numerous secondary genomic alterations. In her work, Soragni also tries to understand the wider picture. “We take this chunk of tumour that comes to us from pathology, and we do not sort for tumour cells,” she says. “So anything which is within that piece of tumour is within our culture system.” This is valuable, because the 3D tumour microenvironment is known to strongly influence how malignancies respond to treatment.
Her team’s work with chordoma has shown how organoids closely resemble the original tumour, and by generating multiple organoids from each tumour, the group was able to test multiple drug candidates. She hopes to use this approach on other rare tumours to match patients with effective existing drugs or identify new, bespoke treatments.
Similar experiments are done with xenograft models, but organoids can offer a much faster and lower-cost solution. Scientists are working towards a future in which such models are generated for each patient at the time of biopsy, then used to develop customized treatments. However, Vivian Li of the Francis Crick Institute in London, who works with organoid models to identify drug targets in gastrointestinal tumours, cautions that this approach needs much streamlining and standardization to reach the clinic. Progress is being made on this front. Soragni’s group has developed a high-throughput workflow for reliably generating tumour organoids and screening them against hundreds of drugs in the space of a week after biopsy5.
An exciting clinical use for organoids sees them as building blocks for lab-grown transplant tissue. This is another focus of the Li group, which has shown that it can cultivate gut organoids that encapsulate the structure and function of segments of the small intestine. The aim is to treat people with intestinal failure. In a proof-of-concept study, her team and collaborators at London’s Great Ormond Street Hospital showed that they could transplant patches of intestinal tissue generated from patient-derived organoids into mice and grow them for up to two weeks6. “We proved that it can function,” says Li. “It can digest complex sugars into simple sugars, it can achieve protein absorption.”
In July, researchers at Japan’s Tokyo Medical and Dental University transplanted gut organoids into a person with ulcerative colitis to repair intestinal wall damage. Li thinks that more extensive transplantation efforts will be challenging, requiring large numbers of organoids and a biocompatible scaffold to produce sufficient quantities of healthy tissue. To this end, her group is working on methods to efficiently generate thin sheets of organoid-derived intestinal tissue that can be grown at a larger scale and then grafted into patients. It might take a few years to translate to the clinic, but Li says she is hopeful that it can be achieved in the next decade.





More News
Bird flu virus has been spreading in US cows for months, RNA reveals
Judge dismisses superconductivity physicist’s lawsuit against university
Future of Humanity Institute shuts: what’s next for ‘deep future’ research?