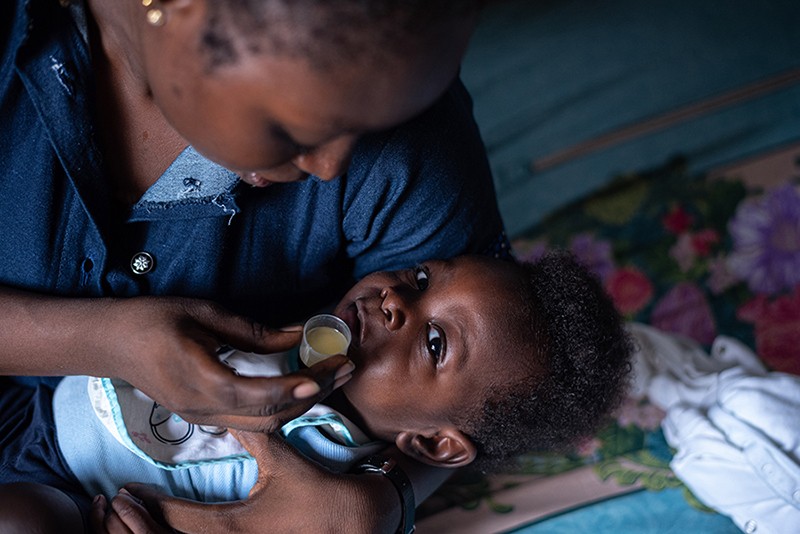
Earlier this year, Deborah Ashom received news that many Nigerian mothers fear. Sean, her six-week-old son, had tested positive for sickle-cell disease. She knew this meant his future might hold bouts of anaemia, infections and painful crises resulting from his c-shaped red blood cells. It would probably also shorten his life — in Africa, up to 80% of children born with the disease die before they turn 5.
Such poor statistics might make screening newborns for the telltale genetic signs seem futile. But Ashom knew it was the right thing to do. The diagnosis, although daunting, will help her to give her son the best possible chance. Sean is now taking penicillin to stave off life-threatening infections such as pneumococcal disease and has been prescribed folic acid, to help replenish his red blood cells and prevent anaemic crises. “There was no point hiding from reality,” says the 33-year-old mother of two.
Sean was the second baby in Nigeria to be diagnosed with sickle-cell as part of a programme to see whether newborn screening can improve these children’s chances of survival. The Consortium on Newborn Screening in Africa (CONSA), funded by the American Society of Hematology (ASH), aims to screen 10,000–16,000 babies per year over the next 5 years in Nigeria, Ghana, Kenya, Liberia, Tanzania, Zambia and Uganda. Children diagnosed with the disorder will, like Sean, be offered medications to manage the disease, and their parents will be educated on how to care for them.
Such interventions can be “truly lifesaving” says Raffaella Colombatti, a paediatric haematologist–oncologist at the University of Padova in Italy. One of the main causes of death in infants with sickle cell, she says, is sepsis. And prevention of sepsis requires administration of penicillin from two months of age. “With newborn screening you can recognize the disease early, implement cheap treatments and clinical supervision early, and educate parents to recognize danger signs early,” Colombatti says.
Contending with cost
About 1,000 children in Africa are born with sickle-cell disease every day. Few are offered top-of-the line treatment such as gene therapy or the anti-sickling drug hydroxyurea, and most will die in childhood. But as other countries have shown, childhood death for this disease is not an insoluble problem. In the United States, mortality in children under 5 with sickle cell fell by more than 60% in 1999–2009 compared with 1979–98 (D. Hamideh and O. Alvarez Pediatr. Blood Cancer 60, 1482–1486; 2013). This was achieved through simple interventions such as early screening, administering penicillin to sickle-cell children and giving them pneumococcal vaccines.
The main reason that such routine screening and treatment has not been rolled out in most African countries is the cost, says Nancy Berliner, a haematologist at Harvard Medical School in Boston, Massachusetts, who co-chairs CONSA. A 2020 report by the Economist Intelligence Unit estimated that screening all newborns in Ghana for sickle cell would cost the country US$6.8 million dollars per year (see go.nature.com/3lwgacg). That is almost 1% of its health-care budget, which is overstretched as it is, particularly with COVID-19 continuing to put pressure on health-care systems. A scientist involved in the CONSA project says that the gold-standard machines used for screening blood for sickle cell cost around $400,000 off the shelf — which is steep even for an oil-producing country such as Nigeria, given that minimum wage in the country is a mere $70 a month.
Through CONSA, participating countries are getting a leg-up from PerkinElmer, a manufacturer of analytical instrumentation in Waltham, Massachusetts. Vanguard CONSA countries such as Nigeria and Zambia purchased screening machines from PerkinElmer at reduced rates, and the company has since agreed with CONSA to donate two machines to each participating country. PerkinElmer is also offering donations and discounts on consumables such as reagents and is training people to use the machines.
However, the pilot programme will cover only a tiny fraction of all babies born with sickle-cell disease in the seven participating countries. The programme’s main role will be to establish the value of newborn screening to governments in each region, says Berliner. “We’re doing a demonstration project, providing funding to get countries started. But the broader success of newborn screening programmes requires a national effort in each participating country that rests with the government,” she says. For this reason, countries had to meet some minimum requirements to join the programme. They needed to have a laboratory in which the screening could be conducted. They needed to identify clinics in which blood could be collected from newborn babies for screening. They also needed to identify a national coordinator to embed screening into local health policies.
In Zambia, which launched its newborn screening programme in April, the national coordinator is Catherine Chunda-Liyoka, who heads the paediatric haematology department at The University Teaching Hospital in Lusaka. Two years ago, her department witnessed the devastation sickle cell can bring, when a child came in with a swollen and blocked spleen. Chunda-Liyoka and her colleagues tried to remove the spleen. It was not enough — the child died from postoperative bleeding and sepsis.
Seeing the boy’s young parents mourn their son was heartbreaking, she says, especially as his death could possibly have been avoided. “We could have diagnosed him much earlier, taught his mum to palpate his spleen, started him on prophylactic antibiotics, antimalarial drugs and folic acid,” she says. “The child and his parents were on my mind when we prepared to launch the screening programme.”
But the disease is not an easy one to tackle. “Like in many other African countries, it’s an invisible disease, with lots of stigma attached to it,” Chunda-Liyoka says. When children die mysteriously, there are often rumours of witchcraft, and partners blame each other for bringing the disease into the family, even though both parents have to be carriers for a child to become sick.
It’s a challenge that’s familiar also to Obiageli Nnodu, who coordinates Nigeria’s CONSA programme. A haematologist with 36 years of experience in the field, she pioneered newborn screening for sickle-cell disease in a handful of the country’s vaccination centres, which have proliferated around Africa over the past 20 years owing to vast immunization drives. Nnodu thinks the stigma will fade if more children with sickle cell live longer and healthier lives. “That’s one of the things early detection can do.”
The data bonus
Although the main aim of CONSA is to show that early screening can save lives in Africa, the project will also generate data that will help scientists to better understand the disease. Most sickle-cell research has been conducted in the United States and other high-income countries, where the treatment capabilities and environmental factors are vastly different from what is available in Africa.
Berliner says data from the programme will allow scientists to map out and study regional differences in sickle-cell survival. Down the line, this could lead to ways to predict which children are likely to have more complications, and prioritize them for treatment and care. The data could also help to explain the relationship between iron deficiency and sickle-cell crises, Berliner says. African countries see higher rates of iron deficiency than do those in the West. Because the rate at which red blood cells sickle is related to the concentration of haemoglobin in the blood, it’s possible that being iron deficient could prevent pain crises in people with sickle-cell disease. But being anaemic is not good for general health, and an important research question for Africa is therefore how best to treat anaemia in regions with a lot of sickle-cell disease.
There is also plenty of anecdotal evidence in Africa that rapid changes in temperature — such as heat waves or cold snaps — can trigger painful crises (S. Tewari et al. Haematologica 100, 1108–1116; 2015), and mothers therefore keep their children with sickle-cell disease warm. But there are “shockingly little” data to back up that link, says Berliner. Long-term studies of groups of people diagnosed with sickle-cell disease at birth will be crucial to investigating such claims, says Ambroise Wonkam, a geneticist at the University of Cape Town in South Africa. It’s important, says Wonkam, to respect knowledge that derives from family experiences in Africa, despite the evidence gaps.
In Zambia, data from the programme will provide a much-needed update to national prevalence data, says Chunda-Liyoka. She says that research conducted throughout the 1970s suggested that carrier prevalence of the sickle-cell gene varied considerably — ranging from 1–5% in the southern parts of the country to 25–30% in the north. But with migration to urban areas and intermarriages, that picture has most likely changed, Chunda-Liyoka says. Knowing where sickle cell is most prevalent can help the government to target those areas for treatment programmes, and can also help it with budgeting, because screening cost varies according to location.
But even with newborn screening, the path for children diagnosed with sickle cell can be arduous. Kehinde Craig, a retired Nigerian physician whose son Olayinka died from sickle-cell disease last year at the age of 41, can attest to that. Over the course of his life, Craig’s son developed many of the complications that can affect people living with sickle cell, including a stroke that put him in a wheelchair. “It was certainly not an easy deal — not on him, or on the rest of the family,” says Craig. But she thinks that diagnosing her son at seven months old, before he became sick, helped her and her family to prepare to care for him and help him to live to middle age.
Meanwhile at six months of age, Deborah Ashom’s son Sean is thriving. “He’s full of strength and energy. If not for the test, nobody would ever suspect he had sickle cell,” says his mother — and the life-preserving measures might never have been taken. She remains anxious about what is to come. “I’m worried. I have to think about his future, what he will experience,” she says. “But at least this way I know how to take care of my child properly.”







More News
Risks of bridge collapses are real and set to rise — here’s why
Keep an open mind on faster-than-light ‘tachyons’ as the source of quantum entanglement
‘Stop the xenophobia’ — South African researchers sound alarm on eve of election