During his 25 years as a nephrologist, Hans-Joachim Anders has travelled to many conferences on kidney disease. On his return, his patients often ask him if he has learnt anything relevant to them. For more than two decades, “I had to tell them no”, says Anders, who is at the Ludwig Maximilian University of Munich in Germany.
But over the past three years, a class of drugs called sodium-glucose co-transporter 2 (SGLT2) inhibitors have completely changed that. Originally designed to treat high blood sugar in people with diabetes, these drugs have also brought hope to people with chronic kidney disease (CKD). SGLT2 inhibitors protect the kidneys and reduce the risk of death from cardiovascular disease — the biggest cause of death for people with CKD.
Anders says SGLT2 inhibitors, also called flozins, have given many of his patients the chance to live dialysis-free for much of their lives. “It has totally changed the paradigm,” he says. By one estimate, an SGLT2 inhibitor called canagliflozin has the potential to delay end-stage renal disease and the need for dialysis by 15 years1.
Part of Nature Outlook: Chronic kidney disease
SGLT2 inhibitors are a staple in many nephrology and diabetes clinics, and understanding of their usefulness continues to expand as data emerge. In 2022, researchers reported promising results from the trial of an SGLT2 inhibitor called empagliflozin2. The phase III trial, called EMPA-KIDNEY, showed that the drug helped to preserve kidney function in people with a wide range of CKD types and severity.
“It’s an exciting time for nephrology,” says Adeera Levin, a nephrologist at the University of British Columbia in Vancouver, Canada, and executive director of BC Renal, an organization that oversees kidney services in British Columbia. Levin, who worked on EMPA-KIDNEY as well as other trials of SGLT2 inhibitors, says her patients get satisfaction from watching the drugs slow down their loss of kidney function on their test results. “They can see the line plateau,” she says. “They get quite excited about that.”
Still, many people with CKD are waiting to find out if SGLT2 inhibitors will be recommended for their particular type of disease. In the meantime, clinicians are prescribing them off-label, providing opportunities to learn who else can benefit from this new drug class.
An unexpected success
The protein SGLT2 sits on the inner surfaces of the kidneys’ million or so winding tubes, known as nephrons. These take the waste that has been filtered out of the blood and carry it to collecting ducts that lead to the urethra and ultimately out of the body. SGLT2 harvests sodium and glucose from the waste moving through the nephrons, and returns them to the blood. Blocking it with the inhibitors reduces blood sugar levels by forcing the sodium and glucose to exit the body in urine instead.
When SGLT2 inhibitors were first approved for use in 2013, Anders says, most nephrologists saw them as just another drug for lowering blood sugar. If anything, there was concern that they might damage people’s kidneys, as some other diabetes drugs had been known to do. That worry didn’t last long — trials testing the drugs’ safety in people with type 2 diabetes showed that, compared with a placebo, SGLT2 inhibitors reduced the likelihood of hospitalization or death from heart failure, cardiovascular events or renal causes3. “Every study that came out, they just seemed to rock,” says Alexander Kula, a nephrologist at the Ann and Robert H. Lurie Children’s Hospital in Chicago, Illinois.
These early signals led to trials specifically designed to detect heart- and kidney-related changes in people with type 2 diabetes and CKD. In the past four years, three of these trials were halted early because the benefits were so clear. In the CREDENCE study4, for instance, canagliflozin reduced the combined risk of end-stage renal disease or death from cardiovascular or kidney-related events by 30%. The drug also significantly slowed the loss of kidney function over two and a half years. “People were becoming a bit cynical about new treatments for kidney disease, about our ability to slow deteriorating kidney function in patients that inevitably get to dialysis,” says David Wheeler, a physician scientist at University College London who worked on CREDENCE. The advent of SGLT2 inhibitors has altered that mindset.
To researchers, including Wheeler, these emerging data suggested that SGLT2 inhibitors might improve kidney health even in people who don’t have type 2 diabetes. So, Wheeler and his colleagues designed a trial that looked for changes in kidney function in a group of people with CKD that had a wide range of causes — not just type 2 diabetes.
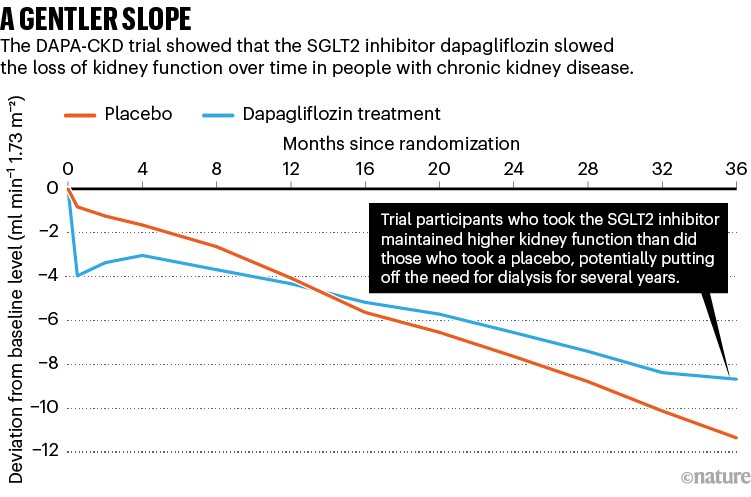
This trial, called DAPA-CKD, showed that regardless of the cause of the disease, an inhibitor called dapagliflozin reduced the risk of end-stage kidney disease by 36% and the risk of hospitalization for heart failure or death from a cardiovascular event by 29%5. DAPA-CKD participants who took dapagliflozin were also 47% less likely to lose half of their kidney function (see ‘A gentler slope’), and significantly less likely to die from any cause over the course of the study. After just two years, the study was stopped because of the obvious benefits.
Source: Ref. 5
In the past few years, trials and observational studies involving tens of thousands of people have reinforced the use of SGLT2 inhibitors outside diabetes — most recently, the EMPA-KIDNEY trial, which included more than 3,000 people with CKD and no diabetes. “After EMPA-KIDNEY, I think we’re done,” says Christos Argyropoulos, a nephrologist at the University of New Mexico’s Sandoval Regional Medical Center in Rio Rancho.
It’s now clear that SGLT2 inhibitors are more than just diabetes drugs. In fact, their effects on the kidneys have little, if anything, to do with lowering blood sugar, and more to do with protecting the organs. Research over the past ten years has shown that, in rats and in humans, sodium and glucose forced to remain inside the nephrons trigger a signal that lowers the flow of blood into the filtration structures of the kidney, the glomeruli6. In kidney diseases, the delicate glomeruli are chronically pummelled by high blood pressure and lose their filtering ability over time. For more than two decades, the only treatment for this problem was a class of drugs called angiotensin-converting enzyme (ACE) inhibitors, which increase the outflow of blood from the glomeruli. Taking SGLT2 inhibitors alongside these drugs decreases the influx of blood and brings the pressure down even further.
Researchers are exploring many other potential mechanisms, most if not all of which lead to reduced cellular stress in the kidneys’ filtering structures. “CKD means the remaining structures are suffering from too much workload — and when you are able to reduce that workload, the kidney lasts longer,” Anders says.
As well as solidifying the evidence that SGLT2 inhibitors work independently of changes to blood sugar, EMPA-KIDNEY also showed that these drugs can preserve kidney function in people who don’t have protein in their urine — a common marker of kidney damage. This is a big step because currently only those with protein in their urine are eligible for SGLT2 inhibitor therapy, even though kidney damage can be present without it. Drug regulators and professional guidelines are likely to incorporate the data from EMPA-KIDNEY and make it easier for clinicians to prescribe the drugs regardless of urine protein levels.
Some people with CKD are being left out of the SGLT2-inhibitor revolution, however. In many cases, Levin says, people who meet the current criteria and should be taking the inhibitors simply aren’t getting them. For a variety of reasons — from cost to their reputation as drugs for lowing blood sugar — SGLT2 inhibitors have been slower to catch on as treatments for CKD than many had anticipated, especially outside the context of diabetes. As the evidence grows for their usefulness in different populations and across various causes of CKD, she says, the drugs could become more accessible.
Spread the benefits
There are also those with kidney disease for whom SGLT2 inhibitors have not yet been approved, but who could still benefit from them. One example is recipients of kidney transplants. Argyropoulos says that he prescribes the drugs off-label for transplant recipients if they have heart failure or cardiovascular disease, but he doesn’t know whether the drugs are helping to prolong the function of the transplanted organs.
Although there are few data on SGLT2 inhibitors for transplant recipients and people with end-stage kidney disease, many studies have started to include people with severely decreased kidney function, defined as a glomerular filtration rate (GFR) below 25 millilitres per minute per 1.73 m2 (normal GFRs lie between 90 and 120 millilitres per minute per 1.73 m2). Researchers at the UK-based pharmaceutical company AstraZeneca and University Medical Center Groningen in the Netherlands are conducting a trial of 1,500 people covering those with severely decreased kidney function, those on dialysis and transplant recipients.
In the few studies involving transplant recipients that have been completed, the drugs had a small impact on some measures, including body weight and control of blood sugar levels. And last November, researchers reported a retrospective analysis of 123 transplant recipients, concluding that SGLT2 inhibition slowed loss of kidney function over the course of one year7.
In theory, children with CKD could benefit the most from SGLT2 inhibitors, but little is known about the effects in this group. CKD in children is often genetically determined and has no specific drug targets, says Francesca Becherucci, a paediatric nephrologist at Meyer Children’s Hospital of Florence in Italy. Because the inhibitors target mechanisms common to many kidney diseases, they could be a great option for kids.
Paediatric nephrologist Petter Bjornstad at Children’s Hospital Colorado in Aurora prescribes the drugs off-label for adolescents with type 2 diabetes, because when the disease starts in childhood, it comes with much higher risks of heart and kidney problems. “They’re in their teens and they have diseases you see in people in their 50s and 60s,” he says. “Their trajectory is gruesome.” Bjornstad says that he considers the drugs to be safe for his teenage patients and he sees clear improvements in markers of kidney function, such as a drop in urine protein levels.
In 2021, the European Medicines Agency approved dapagliflozin for children as young as ten, with the goal of lowering blood sugar in type 2 diabetes. Belgium based Pharmaceutical company Janssen is now testing canagliflozin in 10- to 17-year-olds with type 2 diabetes. And Bjornstad is collaborating with Canadian researchers to test dapagliflozin’s impact on kidney function in children aged 12–18 with type 1 diabetes. Beyond diabetes, however, paediatric data are almost non-existent. Kula is aiming to carry out an observational study to help fill this gap, but there are no clinical trials planned.
Although SGLT2 inhibitors were first developed as drugs for type 2 and type 1 diabetes, in most countries they are no longer approved for the latter because of an increased risk of ketoacidosis — an excess of molecules called ketones in the blood that causes nausea, pain and malaise. The condition requires hospitalization and, rarely, can lead to death. For most people with type 1 diabetes, the blood-sugar-lowering effects of SGLT2 inhibitors are not worth the risk, says David Cherney, a physician scientist at the University of Toronto in Canada.
However, he says, because of the drugs’ impact on heart and kidney health, for people with type 1 diabetes and CKD who are at high risk of end-stage renal disease or cardiovascular diseases, “there may be a huge benefit” to taking SGLT2 inhibitors. In small trials of empagliflozin in people with type 1 diabetes, Cherney and his colleagues found that the drug reduced the amount of protein in urine and helped to preserve kidney function. “All those changes are the same in type 1 and type 2,” he says. “We just don’t have outcome data from large trials to show that.” EMPA-KIDNEY did include 68 volunteers with type 1 diabetes, but analysis didn’t focus on this group.
Ketoacidosis is a risk for people with type 2 diabetes as well, but, Levin says, most, if not all, cases in this group have been related to some other event, such as people starting a very-low-carbohydrate (‘keto’) diet, running out of insulin or having a severe illness or surgery. Argyropoulos says that the risk is easily managed by education, pausing SGLT2 inhibitors when necessary or not giving the drugs to people at very high risk of ketoacidosis.
A layered approach
Argyropoulos was an early adopter of SGLT2 inhibitors for kidney disease, incorporating them into his practice in 2015. Before that, he says, if a person with CKD stopped responding to the standard of care, he had little to offer them besides ramping up doses and starting the countdown to dialysis. “Patients didn’t see much value in attending clinic,” he says.
That has completely changed. Argyropoulos now regularly prescribes SGLT2 inhibitors along with standard-of-care blood-pressure-lowering drugs, such as ACE inhibitors, as well as newer drugs that block genes involved in kidney-damaging inflammation and fibrosis, such as finerenone. After several months on this regimen, people no longer have protein in their urine, their kidney function stabilizes and they gain years of dialysis-free living. He can now discharge patients from his clinic and see them every few years instead of every few months. “I had not been able to do this before all these drugs became available,” he says. “I think this is where the game will be played and eventually won.”
More from Nature Outlooks
A layered approach such as the one Argyropoulos uses is considered safe, but there are few data on how well these drugs work together, which ones add value and which are redundant. That information is sorely needed, Argyropoulos says, so physicians can make the best treatment choices and keep the cost of drugs down. The combination of drugs Argyropoulos uses, for example, adds up to about US$2,000 a month; he’d like to know whether he could get the same results with fewer drugs.
Some companies are beginning to gather data that might help physicians to determine the best combinations for their patients. German pharmaceutical company Bayer, which produces finerenone, is conducting a phase II trial comparing the effect of finerenone in combination with empagliflozin to that of either drug on its own. The trial will measure changes to the amount of protein in urine and kidney function in people with type 2 diabetes and CKD.
“The promise of these combination therapies is so exciting,” Bjornstad says. Some combinations might not only provide additive benefits but also reduce side effects. For example, in rats, SGLT2 inhibitors mitigate fluid retention and oedema caused by a class of drugs used for CKD called endothelin receptor antagonists8. AstraZeneca is now sponsoring two trials of this combination in people with type 2 diabetes or CKD. Even as nephrologists await more data on drug combinations, they already agree that the availability of SGLT2 inhibitors for CKD has breathed new life into their field. “It has given preventative medicine in CKD new energy,” Wheeler says. “It’s made me want to go try out more medicines and find some more that might do this.”






More News
Could bird flu in cows lead to a human outbreak? Slow response worries scientists
US halts funding to controversial virus-hunting group: what researchers think
How high-fat diets feed breast cancer