Around lunchtime on a warm March day in 1999, Kathleen Folbigg went to check on her sleeping 18-month-old daughter and found her pale and unresponsive. Folbigg, alone in her house in Singleton, Australia, called an ambulance while she tried her best to resuscitate the child. “My baby’s not breathing,” she said, pleading for them to hurry.
“I’ve had three SIDS deaths already,” she explained, referring to sudden infant death syndrome — a largely unexplained phenomenon that typically affects infants in their first year, as they sleep.
Around 9 p.m. that night, pathologist Allan Cala conducted an autopsy on the baby, named Laura, at the New South Wales Institute of Forensic Medicine in Glebe, a suburb of Sydney. In his report, he noted no evidence of injury and no medications, drugs or alcohol in her system. He mentioned some inflammation of the heart, possibly caused by a virus, but surmised that it could be incidental. Instead, Cala opined on the improbability of four children in the same family dying from SIDS. “The possibility of multiple homicides in this family has not been excluded,” the report stated.
Four years later, in May 2003, a jury found Folbigg guilty of murdering three of her children — Patrick, Sarah and Laura — and of the manslaughter of her first son, Caleb. Because there were no physical signs of foul play in any of the deaths, the case had rested entirely on circumstantial evidence, including the unlikelihood of four unexplained deaths occurring in one household. Lightning doesn’t strike the same person four times, the prosecutor told the jury.
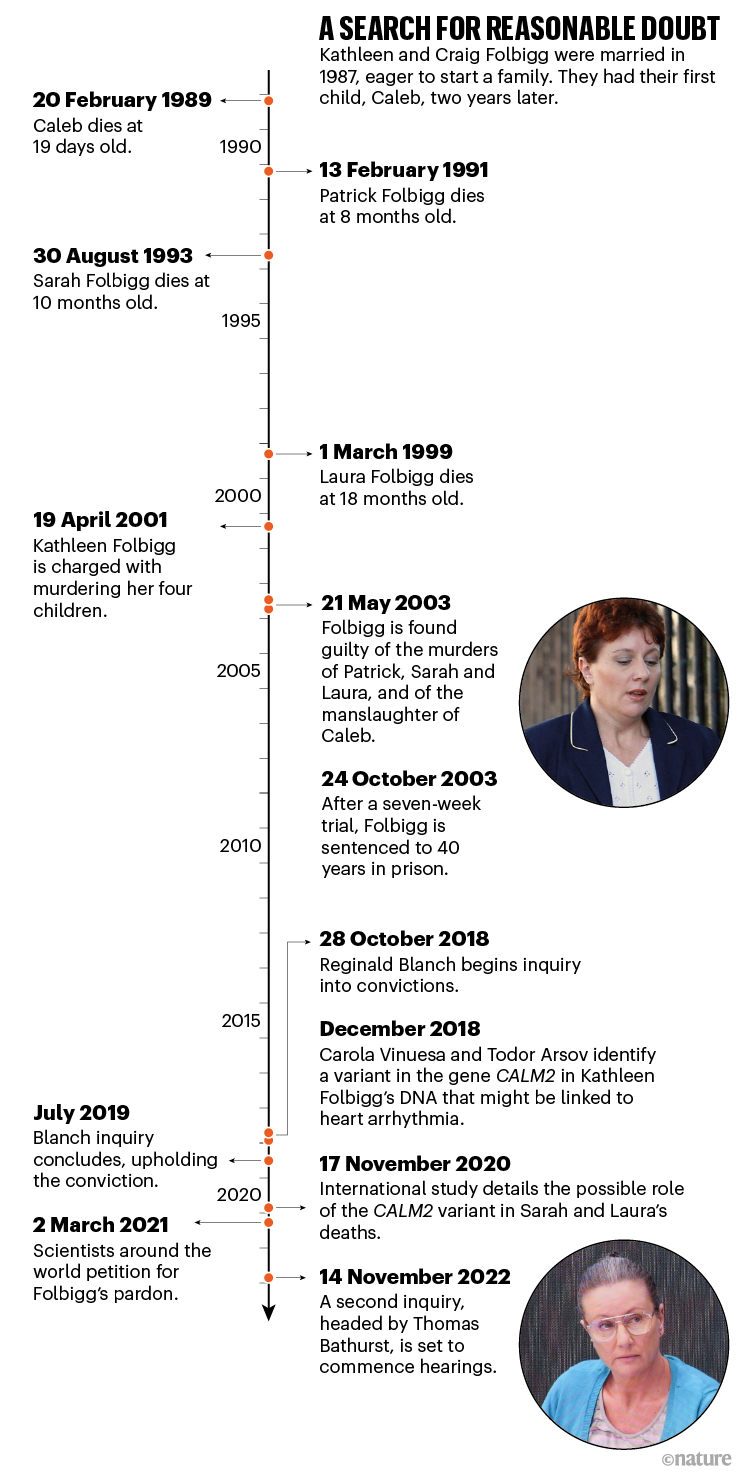
Folbigg was sentenced to 40 years in prison, and became known as Australia’s worst female serial killer. But in 2018, a group of scientists began gathering evidence that suggested another possibility for the deaths — that at least two of them were attributable to a genetic mutation that can affect heart function. A judicial inquiry in 2019 failed to reverse Folbigg’s conviction, but this month, the researchers will present a bolus of new evidence at a second inquiry, which could ultimately end in freedom for Folbigg after nearly 20 years behind bars. More than 90 scientists signed a March 2021 petition arguing for her release on the basis of that evidence.
The controversial company using DNA to sketch the faces of criminals
The inquiry will have to grapple with how science weighs the evidence for genetic causes of disease, and how that fits with the legal system’s concept of reasonable doubt. But it will have help. Thomas Bathurst, the retired judge leading the inquiry who will decide Folbigg’s fate, has granted permission for the Australian Academy of Science in Canberra to act as an independent scientific adviser. The academy will recommend experts to give evidence, and will look at questions asked of those experts to ensure their scientific accuracy.
This will probably present the science more accurately than at the original trial, says Jason Chin, a legal academic at the University of Sydney who studies the way science is used in courts. And this case could have implications for how Australian legal proceedings consider scientific evidence in other cases, says Chin.
Sudden suspicion
Folbigg’s four children died over a period of ten years. Caleb was just 19 days old in 1989 at the time of his death. Patrick and Sarah were 8 and 10 months old, respectively. Soon after Laura’s death, Folbigg was placed under suspicion and eventually stood trial in a case that became a dramatic public spectacle. At the time, multiple SIDS deaths in a single family were viewed with suspicion, particularly against mothers.
That suspicion traces at least in part to Roy Meadow, a British paediatrician who studied child abuse. In 1997, he popularized the idea that “one sudden infant death is a tragedy, two is suspicious and three is murder, unless proven otherwise”. At Folbigg’s trial, this line of thinking clearly influenced some experts’ testimony. And several leading pathologists testified that they had never come across multiple SIDS deaths in one family, implying that it was all but impossible — despite there being such cases in the literature, says Emma Cunliffe, a legal scholar at the University of British Columbia in Vancouver, Canada, who wrote a 2011 book about Folbigg’s case called Murder, Medicine and Motherhood.
In fact, by the time the trial started, scientists had been expressing concern about Meadow’s idea for a number of years, and particularly its use in legal cases. Sally Clark, a mother jailed under similar circumstances in the United Kingdom, had successfully challenged her conviction partly on the basis that Meadow’s arguments were statistically unsound and made unsupported assumptions about the rate of SIDS. The idea was largely discredited by 2003, and Meadow was eventually struck from the UK medical register in 2005 because of misleading testimony he had offered during Clark’s trial.
But Folbigg had other circumstantial evidence weighing against her, says Cunliffe. A selection of diary entries in which Folbigg expressed guilt and remorse about her deficiencies as a mother were presented by the prosecutor essentially as a confession. The prosecution’s case relied heavily on the testimony of her husband, Craig Folbigg, who, on reading the diaries, became convinced that his wife had killed their children. The jury agreed. Several attempts to appeal the decision failed.
Folbigg has always maintained her innocence, however, and has not stopped fighting for her freedom (see ‘A search for reasonable doubt’). In August 2018, after a petition from her lawyers, the attorney general of New South Wales, Mark Speakman, announced that there would be a review into her convictions on the basis of fresh evidence around multiple cases of unexpected death in the same family. This would result in the first inquiry into the conviction.
Credits; Folbigg 2003: David Gray/Reuters/Alamy; Folbigg 2022: Peter Rae/EPA-EFE/Shutterstock
Genetics enters the picture
As part of their preparations for the inquiry, Folbigg’s lawyers approached Carola Vinuesa, a geneticist at the Australian National University (ANU) in Canberra at the time, to sequence and analyse Folbigg’s DNA. The idea was to see whether she carried any mutations that, if inherited by the children, might offer an alternative explanation for how they died. Vinuesa agreed to help. Her colleague, Todor Arsov, a geneticist who lived in Sydney, travelled to the nearby Silverwater Women’s Correctional Centre, where Folbigg was being held, to collect a sample from her.
That December, Arsov joined Vinuesa in her kitchen to scroll through sequence data looking for variants linked to sudden death. Within 20 minutes, they both came across something interesting: a variant in a gene called calmodulin 2 (CALM2).
Humans have three calmodulin genes, encoding identical proteins that bind to calcium and control its concentration in cells, which helps to regulate the heart’s contractions, among other things. Mutations in these genes are extremely rare, but people who have them often have serious cardiac conditions; sudden deaths have been reported. Vinuesa thought the find was worth further investigation. She suggested to Folbigg’s lawyers that they attempt to sequence DNA from the children and their father.
What the data say about police brutality and racial bias — and which reforms might work
By this point, the inquiry was under way, and Vinuesa was asked to join a team of genetics and cardiology experts who would provide advice. In early 2019, the experts met. They included geneticist Matthew Cook, a colleague of Vinuesa’s at ANU, who joined remotely, and three genetics specialists from the state’s health department in Sydney. Jonathan Skinner, a paediatric cardiologist and cardiac electrophysiologist at Starship Children’s Hospital in Auckland, New Zealand, also joined by video link.
Scientists had managed to obtain DNA from the four children. A hospital in Sydney had Sarah’s frozen fibroblast cells from her autopsy in 1993, and the coroner’s court in Glebe had frozen liver tissue from Patrick’s 1991 autopsy. A laboratory in Melbourne sequenced a whole genome from one child’s heel-prick samples, which are routinely collected at birth, and retrieved just the protein-coding portion of the genome from another’s heel-prick card. Craig Folbigg declined to provide DNA.
By agreement, the experts separated into two groups to analyse the Folbigg genomes. Vinuesa and Cook, from Canberra, formed one. The experts from Sydney — genetic pathologist Michael Buckley, clinical geneticist Alison Colley and Edwin Kirk, who specializes in both fields — formed the other, with input from Skinner. (Members of both teams either did not respond to or declined requests for interviews with Nature to avoid jeopardizing their involvement in the upcoming inquiry.)
To decide whether a variant causes disease, geneticists look at various lines of evidence. These include: whether a variant is rare or absent in the population, which would imply that it has been selected against; whether there are any clinical manifestations of disease in people or families who do carry the variant; and whether studies in cells or rodent models confirm that the variant can have an effect on protein function and health.
Each of these kinds of evidence can be used to score a variant using a five-tiered scale created by the American College of Medical Genetics and Genomics (ACMG), ranging from benign to pathogenic1. In the middle of the scale are ‘variants of uncertain significance’, a messy classification that neither clears nor implicates a given mutation in causing disease.
If the inquiry found that a genetic variant in one or more of the children was ‘likely pathogenic’ or ‘pathogenic’, it could raise enough reasonable doubt about Folbigg’s guilt to overturn her convictions, says Cunliffe.
Kathleen Folbigg, pictured here in 2004, has spent nearly two decades in prison.Credit: Anita Jones/Fairfax Media via Getty
Both teams started with a big-picture approach, using bioinformatics tools to scan the genomes for rare variants. These alone are not a reason for concern or a sign of disease — most rare variants are harmless — but they’re a good place to start when looking for the cause of a potential genetic condition. The Sydney team found 279 among the 5 Folbiggs. By filtering for genes linked to cardiac diseases, respiratory disorders or sudden death, they whittled the list down to just nine, each found in different combinations in the four children and their mother. Within that list was the variant in CALM2 that Vinuesa had identified. It was present in Folbigg and her two daughters, Laura and Sarah.
Vinuesa and Cook in Canberra went through a similar process using their own bioinformatics tools. Overlap between the two teams’ findings was good: both identified CALM2 and a variant in the gene MYH6. The Canberra team also highlighted a mutation in a third gene, IDS, which is involved in a metabolic disease called Hunter syndrome. This can cause seizure and death, and was found in one of the boys, Patrick.
A more-inclusive genome project aims to capture all of human diversity
But when the teams issued their reports in March 2019, they differed in how they classified the rare variants. Although the Canberra team suggested that both the CALM2 and IDS variants were ‘likely pathogenic’, the Sydney group concluded that none was.
In April, the hearings started. Over three gruelling days, genetics and cardiology experts were quizzed by the lawyer assisting the inquiry. The two teams ultimately agreed that the variants in IDS and MYH6 were of uncertain significance and unlikely to be responsible for the children’s deaths. But the two groups could not agree on how to classify the variant in CALM2.
Vinuesa and Arsov, who was involved in the Canberra team’s report, told the hearing that the classification of ‘likely pathogenic’ was based on several ACMG criteria: the variant seemed to be completely new because it was absent in population databases at the time (it has since turned up on CALM3 in one database). And computer simulations predicted it would be damaging to the function of the protein it encodes. The team also argued that there was a plausible pathway to explain how the variant triggers sudden deaths.
But members of the Sydney team challenged this classification, in part because Folbigg was apparently healthy even though she had the variant. Vinuesa argued that Folbigg’s health status wasn’t entirely known. She also explained that it was common for some people with a disease-causing variant not to show obvious signs or symptoms.
Skinner, the cardiologist, agreed that this was the case with some inherited heart conditions. He also agreed that mutations in calmodulin genes do produce life-threatening arrhythmias — irregular heart rhythms. But he told the inquiry that the arrhythmias “don’t seem to present in an infant who’s quietly asleep”. Moreover, he added that in the literature, “there’s not a single case of a sudden death under the age of two”.
A disagreement unresolved
After the hearings had ended, but before the head of the inquiry, former judge Reginald Blanch, had released his findings, Vinuesa e-mailed several scientists who work on calmodulin hoping they could help to settle the disagreements. Peter Schwartz, a cardiologist specializing in arrhythmias of genetic origin, wrote back almost immediately. Schwartz, at the Italian Auxological Institute in Milan, was on the team that had found a link between mutations in calmodulin and sudden death in childhood2. In 2015, he had helped to establish a registry of people with known pathogenic mutations in the CALM genes, called the International Calmodulinopathy Registry3.
Schwartz told Vinuesa that he and colleagues had just published a paper4 that mentions a family with a mutation at the same location as the Folbigg variant, but in a different version of the calmodulin gene, CALM3, and with a different amino-acid substitution. In that family, a 4-year-old boy had died suddenly, and his 5-year-old sister had had a cardiac arrest but survived. Their mother, whose health seems unaffected, is mosaic for the variant, meaning that she carries it in only some of her cells. Vinuesa understood the significance — a new variant that results in an amino-acid change at the same location of a CALM gene is considered strong evidence in the ACMG guidelines. In his letter to Vinuesa, Schwartz wrote: “My conclusion is that the accusation of infanticide might have been premature and not correct.”
Vinuesa sent Schwartz’s letter and the new paper to Blanch, and lawyers sent it on to the Sydney team. But the group of geneticists remained unconvinced. In their reply, they wrote that although the discovery of the similar mutation in another family “has a significant impact on the likelihood” of its pathogenicity, that did not mean it caused the Folbigg girls’ deaths, particularly in light of the fact that their mother is alive and seemingly healthy. For the variant to have caused the deaths of Sarah and Laura would require an “exceptional clinical scenario”, which is “outside the range that has previously been reported in association with variants in this group of genes”, the team wrote. “Our classification of this variant remains that it is a variant of uncertain significance,” they concluded.
Craig Folbigg testified against his wife Kathleen over the deaths of their four children.Credit: Dallas Kilponen/Fairfax Media via Getty
The disagreement between the two teams partly reflects the modern history of clinical genetics. The rapid pace of genomics research over the past two decades powered an exuberant search for pathogenic mutations. The medical literature is littered with papers that claimed to have identified dangerous gene variants that later turned out to be harmless. “It became a bit of a wild west,” says Hugh Watkins, a cardiologist at the University of Oxford, UK, who studies genes that cause sudden cardiac death.
Scientists would link a gene to a certain disease because a newly found variant didn’t show up in a small group of healthy people. Then they would use biochemical assays to show that the variant had some effect on the protein — not even necessarily linked to disease — and the paper would practically write itself. “A lot of us in the field were sceptical that there just wasn’t enough evidence, but these papers were quite easy to publish,” says Watkins.
The reckoning for this type of publication came roughly a decade ago, with the arrival of extremely large databases containing tens of thousands of genomes. Suddenly, it became clear that many supposedly deadly variants were actually relatively common in the population, and were therefore likely to be benign. “At the time, we didn’t imagine all the variation in our genome,” says Morten Salling Olesen, a molecular biologist specializing in genetic cardiac disorders at the University of Copenhagen. As a result, declaring a variant pathogenic now requires a fairly high bar of proof, says Watkins.
What sets the three calmodulin genes apart is that variations in them are very rare in population databases. Of all the highly conserved genes in evolution, “they’re a poster child”, says Watkins. All vertebrates have exactly the same calmodulin sequences. “That is evolution’s way of saying they’re important,” says Salling Olesen.
When scientists published details of the first mutation in 2012, it was eye-opening5. “People thought such mutations would probably be so severe that you would never have a live birth,” says Ivy Dick, an electrophysiologist at the University of Maryland School of Medicine in Baltimore.
Reform predictive policing
In the past few years, the view has evolved again. In large population databases, a small number of seemingly healthy people have calmodulin mutations, albeit in different locations to known pathogenic variants. And it is possible for Folbigg to live with a pathogenic mutation while her daughters might have died from it. “That’s why it’s so fricken complicated,” says Walter Chazin, a structural biologist at Vanderbilt University in Nashville, Tennessee, who studies calmodulin.
In the end, it was left to Blanch to make a decision on the evidence presented by the Canberra and Sydney teams. In his report, released in July 2019, he wrote, “I prefer the expertise and evidence of Professors Skinner and Kirk and Dr Buckley.” He concluded that he was in no doubt about Folbigg’s guilt.
Vinuesa found this conclusion “bewildering”. In a speech she later gave to a gathering of scientists and lawyers, she complained of the legal system’s reliance on “intuition” to inform decisions.
Schwartz, too, was perplexed. In a June 2021 letter to the president of the Australian Academy of Science, he wrote of his concern that Blanch and a subsequent appeal court had argued that Sarah and Laura Folbigg’s deaths did not fit with what is reported in the literature. He countered that there were currently four cases in the calmodulin registry of sudden cardiac deaths or arrests in children under three while they were asleep. The two Folbigg girls “reflect the natural variability associated with these conditions”, he said.
The inquiry’s struggle with scientific evidence did not surprise Cunliffe. When courts are presented with contradictory scientific perspectives, they tend to pick sides, preferring evidence from one expert, she says, and ignoring the uncertainty. Nevertheless, Cunliffe says she finds it “astonishing” that scientists have a possible explanation for how the children died, and yet the court “doubled down on Folbigg’s guilt”.
Some of that undoubtedly comes down to the contents of Folbigg’s diaries and the evidence she gave about them at the inquiry, which Blanch said strongly influenced his decision. For instance, in November 1997, two months after Laura was born, Folbigg wrote that she thought she handled Laura’s crying better than she did Sarah’s. “With Sarah all I wanted was her to shut up. And one day she did,” she wrote. Another entry read: “I feel like the worst mother on this earth. Scared that she’ll leave me now. Like Sarah did. I knew I was short-tempered & cruel sometimes to her & she left. With a bit of help.” Blanch concluded in his report that Folbigg’s diaries were “virtual admissions of guilt for the deaths of Sarah, Patrick and Caleb, and admissions that she appreciated she was at risk of causing similarly the death of Laura”.
A new inquiry
With the latest inquiry under way, Bathurst, who will preside over the proceedings, has stated that he intends to form his own opinion about the evidence. At the first hearings next week, he will consider the functional genetic findings gathered by scientists since the first inquiry, which they say demonstrates that the CALM2 variant is pathogenic.
One of those researchers is Michael Toft Overgaard, a protein scientist at Aalborg University in Denmark, who was part of the team that discovered the first mutation in a calmodulin gene in 2012.
After the first inquiry, Vinuesa e-mailed Overgaard and asked whether he could perform a functional assay to determine the cellular effects of the Folbigg variant. Overgaard wasn’t familiar with the case, but was drawn to the idea of seeing “another piece in the puzzle to try and figure out how calmodulin works”, he says.
Overgaard asked postdocs Helene Halkjær Jensen and Malene Brohus to do the lab work. Everything about the project was secretive: even other researchers in their lab didn’t know about it. “We had a folder on our computer called CSI,” says Jensen, a reference to the popular US television drama about crime-scene investigators.
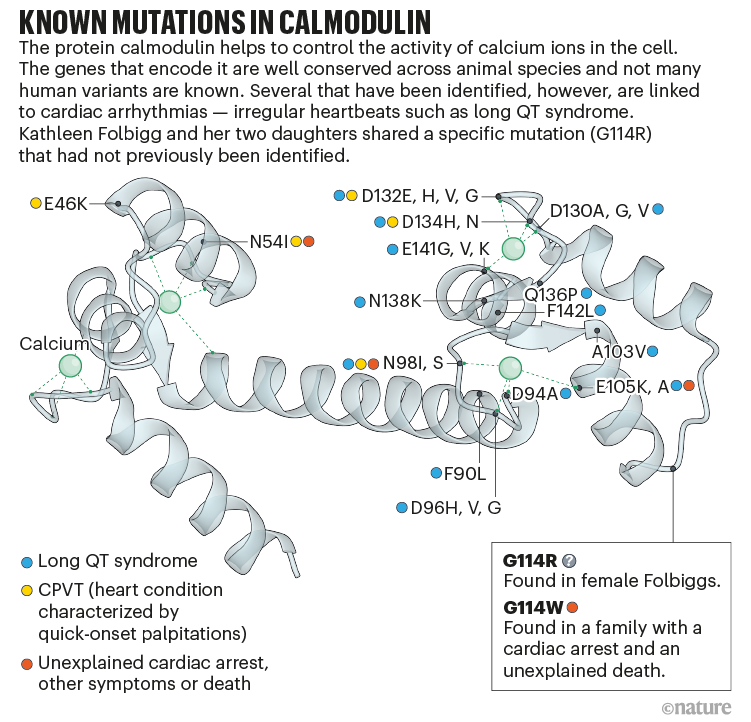
Jensen and Brohus spent weeks painstakingly making calmodulin proteins with the Folbigg mutation, known as G114R, in which the amino acid glycine (G) at the 114th position of the protein is replaced with an arginine (R). For comparison, they created proteins with two other calmodulin variants known to cause severe arrhythmias, G114W and N98S (see ‘Known mutations in calmodulin’).
Source: R. Chattopadhyaya et al. https://doi.org/10.2210/pdb1CLL/pdb (1992); Ref. 4
One of the first things they discovered was that the G114R variant cannot latch onto calcium effectively. The team thought this was important, because the effect was similar to that seen with the other two deadly variants. Further experiments revealed that G114R impairs how calmodulin attaches to two crucial channels that control the movement of calcium into the cell. Jensen says the results were compelling, but the team knew the most convincing evidence would be to show exactly how these channel impairments looked in a cell.
For that they asked Dick, who studies the protein CaV1.2, a channel that shepherds calcium into the cell. Calmodulin triggers this channel to close once enough calcium has entered. Overgaard asked Dick to look specifically at whether the mutation impaired the closure of CaV1.2. Dick had never heard of the Folbigg case, but to prevent her team from introducing any bias, she relabelled dishes of cells to hide their provenance. Sure enough, the Folbigg variant delayed the channel closure, letting extra calcium into the cell. “That’s what we know to be one of the signatures of a pathogenic calmodulin mutation,” Dick says.
But that wasn’t the only effect the variant had. Wayne Chen, who studies calcium channels at the University of Calgary in Canada, was asked to conduct similar experiments on a ryanodine receptor, a channel that controls the release of calcium into the cell from intracellular stores. As it does with CaV1.2, calmodulin binds to ryanodine receptors and triggers the channels to close. This prompts the heart muscle to relax. When Chen’s team expressed the G114R variant in human cells, that channel had trouble closing, too. The combined effect of the variant on both channels will increase calcium in the cell, says Dick, which increases the chance of arrhythmia. “If you asked me, ‘Would this mutation be likely to cause sudden death?’, I would say somebody with this mutation is at very high risk of that,” she says.
A radical revision of human genetics
In November 2020, the international team published its findings in the journal EP Europace6. The researchers concluded that the mutation that the girls carried set the stage for a fatal arrhythmic event that could have been triggered by an infection. Both girls reportedly had respiratory illnesses before their deaths, and the heart inflammation in Laura’s autopsy now looked more relevant than ever.
Many scientists who spoke to Nature find the functional evidence persuasive. Watkins, for example, says the team used sophisticated assays that “faithfully” reflect how the variant impairs crucial functions in the heart cell.
Salling Olesen cautions that functional effects don’t necessarily equal disease. For instance, the Folbigg variant might have only a small effect on heart function, he says. “It’s hard to know.”
But Watkins says: “With a variant that no one else has seen, I don’t think it could be more convincing than what they’ve got.” He adds, “The case that this is plausibly a cause of sudden death in early childhood is strong.”
Enter the academy
The 2020 study prompted the highly respected Australian Academy of Science to get involved. Its chief executive, Anna-Maria Arabia, was already interested in seeing the academy advocate for better representation of science in legal proceedings when she heard from Vinuesa after the first inquiry. Once the EP Europace paper was published, the academy and Folbigg’s lawyers used it as the basis of a petition calling for the governor of New South Wales to pardon Folbigg. More than 90 prominent scientists signed the letter, sent on 2 March last year — among them Nobel laureates Elizabeth Blackburn and Peter Doherty, along with experts in paediatrics, cardiology and genetics. Arabia says not one person she approached knocked her back. “That’s how convincing this paper is,” she says.
Hearings for the inquiry will begin on 14 November, and scientists and clinicians from around the world will be called to give their opinions on the evidence. Although the hearings will focus on the new genetic findings, experts will be called to discuss other aspects of the case, such as the boys’ deaths. And, early next year, psychologists have been scheduled to give evidence about the diaries. Although the documents helped to secure Folbigg’s conviction, some psychologists have challenged the way that they were interpreted.
The hearings will be a chance to test the academy’s role as an independent scientific adviser. So far, commissioner Bathurst has given the academy a broad scope. Arabia says the academy will be giving advice on the most appropriate experts, ensuring questions asked by lawyers are scientifically accurate and that there are robust scientific discussions. “That was missing in the last inquiry,” she says.
Beyond the Folbigg case, the involvement of the academy could set the stage for a new era of science in legal proceedings. A common criticism of the use of expert witnesses is that lawyers are ill-equipped to know the most appropriate people to call, something the academy hopes to change. “It’s an interesting sign of the growing openness to scientific expertise in the Australian court system,” says Cunliffe.
But Chin warns that the process could still be open to bias. Scientists “have an interest in their theory being the dominant one”, he says. Cunliffe, who thinks that Folbigg was wrongfully convicted, agrees that professional associations can be political, too, and so allowing them into the courtroom is unlikely to be a panacea. Still, she says, the academy’s involvement “is a very good thing for the Folbigg inquiry”.
The outcome of the inquiry won’t be known for months. But if Bathurst finds that the genetics offer a reasonable explanation for the deaths of the Folbigg girls, it could eventually spell release for their mother, about five years before she would have been eligible for parole. Folbigg wrote in 2006 that she just wants the truth to be uncovered. “That day I shall not gloat, or say, ‘I told you so’ I’ll simply cry and keep crying all the tears that are due to me.”














More News
Author Correction: Stepwise activation of a metabotropic glutamate receptor – Nature
Changing rainforest to plantations shifts tropical food webs
Streamlined skull helps foxes take a nosedive