Health workers have started to vaccinate children against malaria in three countries.Credit: Joseph Oduor/AP via Alamy
In 1967, Austrian–Brazilian immunologist Ruth Nussenzweig made a momentous discovery. By blasting malaria parasites with radiation, she could weaken them. When injected into mice, these feeble parasites were unable to replicate and cause disease. But they could prompt an immune response.
The discovery was the beginning of what would become a more than five-decade quest to develop a malaria vaccine — a feat that seemed impossible at times. “There was just a general pessimism that there would ever be a malaria vaccine,” says Patrick Duffy, head of the Laboratory of Malaria Immunology and Vaccinology at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland, who started working on the problem in the 1990s.
Part of Nature Outlook: Malaria
The World Health Organization’s decision to recommend the first malaria vaccine in 2021 — known as RTS,S — has brought about a seismic shift. “My gosh, there’s so much enthusiasm,” Duffy says. There’s also a lot of room for improvement.
RTS,S is currently being administered to children in three countries and is expected to save more than 20,000 lives each year. But it’s far from perfect. The vaccine is administered as four shots to children from 6 weeks old, and it confers only modest protection, preventing about 50% of cases initially and waning from there. “We definitely need new tools,” says Ashley Birkett, director of the malaria vaccine initiative at the non-profit organization PATH in Seattle, Washington.
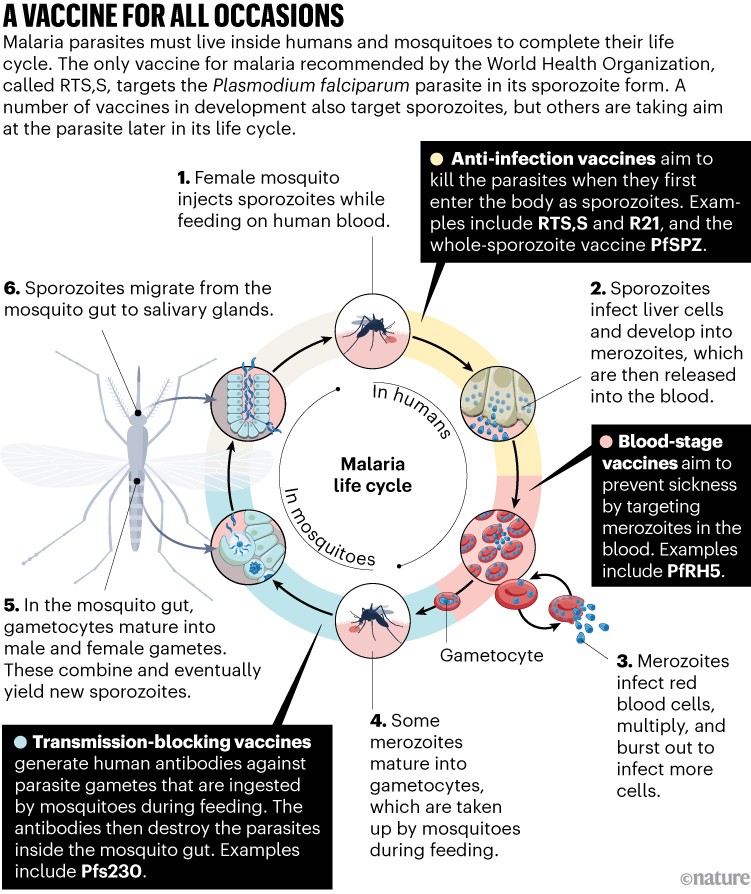
In September 2022, the WHO published a wish list for the ideal malaria vaccine — one that is safe, that can be administered in a single dose and that is 90% effective. “That’s a very, very high goal,” says Mary Hamel, head of malaria vaccines at the WHO in Geneva, Switzerland. But vaccine developers are striving to get closer to it. There are numerous malaria vaccines in development aimed at Plasmodium falciparum, the deadliest and most-studied malaria parasite. Some, like RTS,S, aim to prevent the parasite infecting the liver. Others target the parasite at different stages of its life cycle in an effort to curb illness and block transmission. Many of these vaccines have been in the works for years, but researchers are closer than ever to the next malaria shot.
Decades in development
Developing a vaccine for malaria is no easy feat. The parasite has a complex genome that contains the blueprints of more than 5,000 proteins, each of which is a potential target for a vaccine. What’s more, the parasite must live inside two different hosts to complete its life cycle, and undergoes vast changes as it does so.
The parasite begins its journey in a mosquito’s gut, and then migrates to the insect’s salivary glands. When an infected insect bites a person, the parasites are injected into the bloodstream in the form of worm-shaped sporozoites. These work their way into liver cells, where they mature and replicate into tens of thousands of smaller merozoites before returning to the bloodstream. The merozoites then infect red blood cells and replicate once again, before bursting out and causing a fever. Some merozoites transform once again into a sexual form that the mosquito will take up during its next blood meal, ready to repeat the cycle.
At each stage, the parasite changes radically. “The parasite as it passes through the liver is very different from the parasite as it passes through the blood stages. And that’s very different from the parasite that passes through the mosquito,” Duffy says. Vaccine developers have to choose which form to target.
RTS,S goes after sporozoites. Its aim is to generate an immune response that can eliminate them before they mature into their next form, stopping the infection before symptoms develop. The vaccine consists of a portion of the hepatitis B virus, which serves as a scaffold, fused to a segment of circumsporozoite protein — the most abundant protein on the sporozoite’s surface. These self-assemble into virus-like particles in yeast cells. The particles are then combined with an immune-boosting adjuvant, AS01, which contains compounds from the bark of a Chilean tree and a modified bacterial toxin. The shot triggers the body to make antibodies against circumsporozoite protein. When these antibodies encounter a sporozoite, they tag it for destruction.
Despite the excitement of finally having an approved vaccine for malaria, the efficacy of RTS,S is underwhelming. In trials in children, the vaccine prevented about half of malaria cases for one year, but protection dropped to less than 40% in year two1. And to achieve even that level of protection, four doses are required. “Malaria is this huge problem and RTS,S is filling a gap,” says Stephen Hoffman, chief executive and scientific officer at biotechnology company Sanaria in Rockville, Maryland, which is developing a vaccine of its own. But “it’s clear that it’s really, at best, modestly protective”.
At the University of Oxford, UK, researchers led by vaccinologist Adrian Hill are working on a vaccine called R21, which targets the same protein as RTS,S. R21 also uses a hepatitis-B-derived scaffold, and contains an adjuvant called Matrix-M, which includes the same tree bark compounds as AS01. The key difference is that, in RTS,S about 80% of the hepatitis B protein fragments are not fused to circumsporozoite; R21, by contrast, contains only the fusion protein.
This means that a dose of R21 contains more circumsporozoite protein per microgram than RTS,S, which might give it “a little more muscle” says Stefan Kappe, an infectious-disease researcher at the University of Washington, Seattle. However, differences in the amount of each vaccine given in one dose — 5 micrograms for R21 and 25 micrograms for RTS,S — mean that they probably deliver roughly the same amount of the protein in total, Duffy says.
The sporozoite form of the malaria parasite.Adapted from A.S. Orfano et al. Malar. J. 15, 394 (2016).
In terms of efficacy, R21 does seem to perform a little better than RTS,S. In a phase II trial in Burkina Faso, R21 provided up to 77% efficacy against clinical malaria over a year2. Volunteers receiving a booster had about 70% protection over the following year. Some researchers have speculated that R21’s seemingly greater efficacy might be because the vaccine was administered just before the rainy season in places where malaria is highly seasonal, possibly giving it an advantage. However, last year, Hill presented preliminary data from a phase III trial showing efficacies of more than 70% in both seasonal and non-seasonal malaria sites.
The WHO started to review the R21 data in March to determine whether to give the vaccine the green light, and that will probably continue for many more months. “It is early to provide a specific time line,” says Hamel. With demand for a malaria vaccine likely to outstrip supply for years, Nigeria and Ghana have already approved the vaccine, but it’s not yet clear when it will be rolled out.
A complete approach
At Sanaria, scientists are taking another tack. Rather than singling out one sporozoite protein, they’re delivering whole, live sporozoites. The key advantage of a whole-parasite vaccine, says Kappe, is that it provides a multipronged immune response against “dozens, if not hundreds of proteins”. That might allow the vaccine to hold up better as the parasite evolves. “The parasite cannot change 500 proteins at the same time,” he says.
To ensure that people who receive the whole sporozoites don’t develop malaria, the researchers either weaken the parasites with radiation or administer them alongside antimalarial drugs — a strategy called chemoprophylaxis vaccination. These vaccines offer high protection when people who have never had malaria are infected in a controlled setting. In one small trial in which chemoprophylaxis was used, researchers achieved 100% protection, also known as sterilizing immunity3. This is a first for any malaria vaccine. “It shows the level of protection that you can get,” Duffy says.
In the field, the results have been less impressive. A vaccine composed of irradiated sporozoites, called PfSPZ, showed little lasting efficacy in a study of around 300 infants in Kenya4. That could be because of the young age of the study participants and their immature immune systems. Another possible explanation is that malaria infection at the time of vaccination blunted the vaccine’s effect. Across all six field trials of PfSPZ that Sanaria had conducted prior to a presentation at the 2021 meeting of the American Society of Tropical Medicine and Hygiene, only the four in which participants were treated for malaria before receiving the vaccine showed efficacy. The vaccine also showed good efficacy in an unpublished trial that included 300 women, over half of whom became pregnant after being immunized. Participants received antimalarial drugs and then either a high or low dose of PfSPZ. The vaccine showed substantial efficacy over the first two years. “It can be durable in a way that the other vaccines have not been so far,” Duffy says.
Rather than administering live sporozoites in concert with antimalarials as these trials did, the vaccine that Sanaria hopes to bring to market will contain sporozoites that have been genetically altered to prevent them from developing past the liver stage. Kappe and his colleagues have created a sporozoite that lacks two genes that are crucial for late-stage development in the liver. Unlike irradiated parasites, these genetically-attenuated parasites can replicate and develop for six days before dying. “The longer you let the parasite develop in the liver, the better the immune response against it,” Kappe says.
“We’re still waiting to see whether the promise of whole sporozoite vaccines can match RTS,S and R21,” Kappe says. Even if they do, many challenges remain. The vaccine must be kept cold using liquid nitrogen and administered intravenously, which complicates its use. In addition, the sporozoites for these vaccines currently have to be extracted by hand from the salivary glands of mosquitoes. Sanaria is working on an in vitro process that would eliminate the need for this delicate work, but it still has much to do to realize large-scale production of sporozoites for mass vaccination.
In the blood
The aim of sporozoite-targeting vaccines is to prevent infection. If the vaccine works, the parasite never has a chance to explode into the blood and cause the symptoms that make malaria so deadly. “The problem is that if one single sporozoite slips through the net and comes out into the blood, then you will get clinical disease,” says Simon Draper, a vaccine researcher at the University of Oxford.
More from Nature Outlooks
In the 1970s, researchers began taking aim at the blood stage of the parasite, the merozoite. A blood-stage vaccine wouldn’t be able to prevent infection, but it might keep numbers low enough to prevent illness. For years, this seemed out of reach. Researchers conducted more than 30 trials of blood-stage vaccines between 2000 and 2015 and found very little evidence of efficacy. The targets chosen either had too much genetic variability, says Draper, or they were redundant. “It’s a bit like having a spare key,” he says. “If you knocked one key out, the parasite had backup after backup.”
But about a decade ago, researchers discovered PfRH5 — a protein that Draper thinks could be the merozoite’s Achilles’ heel5. PfRH5 binds to a receptor on the surface of red blood cells and helps the parasite to gain entry. “We have a highly conserved target, and an essential one,” he says. “There’s no spare key to the lock.”
The vaccine Draper and his colleagues developed combines PfRH5 produced in fruit fly cells with an adjuvant, currently Matrix-M. In a small study published in 2021, Draper and his colleagues tested different doses and timings of administration in healthy adults6. When these volunteers were deliberately infected, the best regimen slowed growth of the parasite by 20%. In field trials in Tanzania, the vaccine induced high antibody levels in children aged 6–11 months7. “The antibody levels in the infants are much higher than what we see in the adults,” Draper says. “That’s been a surprise and a good news story.” A phase II efficacy trial in Burkina Faso launched in April.
Elimination goals
Most vaccines aim to reduce malaria deaths and illness. But to eradicate malaria altogether, scientists will also have to find a way to stop transmission. Sporozoite- and merozoite-targeting vaccines could help to reduce transmission by preventing infections or reducing the number of parasites in the blood. Duffy, however, is working on an altogether different vaccine that makes transmission its main aim — one that can destroy the malaria parasite inside the mosquito.
Inside people, a small subset of merozoites differentiate into male and female gametocytes, which the parasite needs to complete the sexual stage of its life cycle. When a mosquito feeds on an infected person, it ingests red blood cells that contain the parasites’ gametocytes. These gametocytes emerge as gametes in the mosquito’s gut, mate, and eventually give rise to fresh sporozoites ready to infect the next person.
The idea behind Duffy’s vaccine is to take out the gametes by stimulating the human immune system to generate antibodies against a protein called Pfs230 that gametes display on their surface. A feeding mosquito will then take up not just the gametocytes, but also those antibodies. When gametes emerge from red blood cells in the mosquito’s gut, the theory goes, the antibodies will be there to destroy them before they can complete their development (see ‘A vaccine for all occasions’).
Credit: Alisdair MacDonald
Duffy and his colleagues say that they have found that this kind of vaccine can reduce transmission in the field. In their unpublished study in Mali, the researchers allowed malaria-free mosquitoes to feed on people when malaria transmission was high. They found that mosquitoes that fed on vaccinated people were less likely to be infected — the vaccine substantially reduced the number of transmission events.
Giving people a shot to destroy cells inside mosquitoes is “a little bit unorthodox of a vaccine concept,” Duffy says, but blocking transmission is crucial. “A transmission-blocking vaccine probably is going to be most beneficial for elimination efforts or even control efforts.”
Malaria eradication is likely to require a combination of vaccines that hit the parasite at multiple points in its life cycle. “It does feel like we’re moving to an era of combining these different activities and also seeing how they can work together,” Duffy says. Draper is already working with Hill to test R21 and PfRH5 together in a small group of children in Gambia. “We’re excited about that,” Hill says. “If we can get 75% efficacy with R21, then adding an RH5 might bring you up towards 90%, even beyond.” PATH is also interested in this approach, Birkett says. “In the near term, that’s the opportunity I’m probably most optimistic about.”








More News
Author Correction: Stepwise activation of a metabotropic glutamate receptor – Nature
Changing rainforest to plantations shifts tropical food webs
Streamlined skull helps foxes take a nosedive