After Ana Castillo underwent a double mastectomy two years ago, she had one question for her doctors: “What’s the next step?”
Castillo carries an altered form of a gene known as BRCA2 that makes her 3–5 times more likely than the general population to develop breast cancer, and between 9 and 14 times more likely to get ovarian cancer1. The removal of both her breasts drastically reduced Castillo’s breast cancer risk. To address her risk of ovarian cancer, her doctors at MD Anderson Cancer Center in Houston, Texas, suggested two possible courses of action. She could have her ovaries and fallopian tubes taken out at around age 45 — the standard risk-reducing surgery for people who carry mutations in BRCA2 or other ovarian cancer risk genes. Or, Castillo could have just her fallopian tubes removed now, at the age of 36, and keep her ovaries for another 10 years.
This second possibility is the result of a revolution that has occurred over the past two decades in the understanding of the origin of ovarian cancer. It’s now widely accepted that most, if not all, cases of the most common type of ovarian cancer, known as high-grade serous ovarian carcinoma (HGSOC), arise in one of the two fallopian tubes that transport eggs from the ovaries to the uterus.
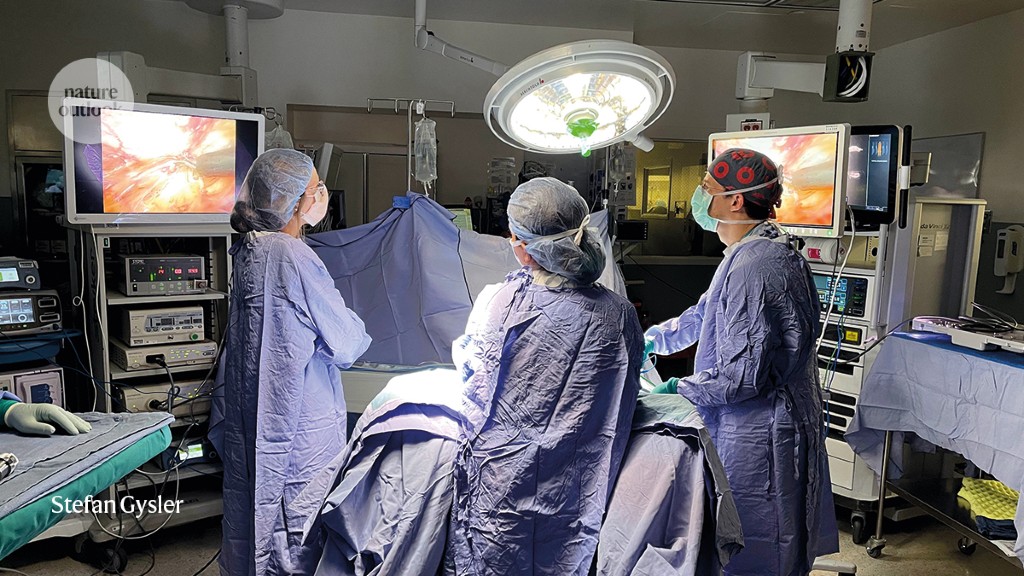
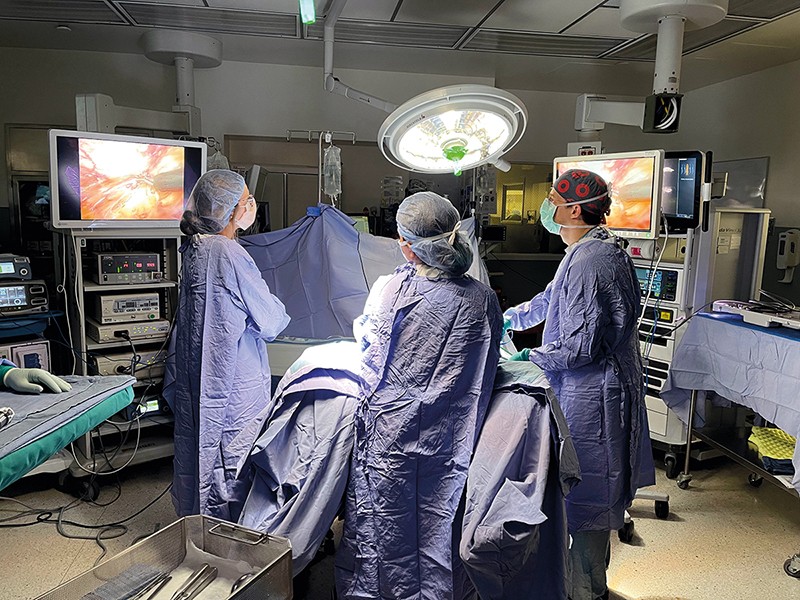
“This has great clinical import,” says David Huntsman, a gynaecological oncologist at the University of British Columbia in Vancouver, Canada. “It has created a tremendous ovarian cancer prevention opportunity.” It means that people such as Castillo might have new options for risk-reducing surgery that have fewer side effects than the conventional approach. Removing the ovaries triggers immediate menopause: a sudden loss of oestrogen that can bring on night sweats, hot flushes and reduced libido, and can increase the risk of heart disease and osteoporosis. The effects hit all at once, and possibly many years earlier than natural menopause would have occurred.
Clinical trials are under way to test the cancer-prevention efficacy of removing the fallopian tubes first and the ovaries later. What’s more, a clearer understanding of ovarian cancer’s origin could lead to benefits not only for people with genes that elevate their risk of ovarian cancer, but also for the much larger majority of people with ovarian cancer who do not have those genes. It might even lead to much needed progress in detecting the deadly disease at an earlier and more treatable stage.
Missing links
For more than a century, physicians and scientists assumed that ovarian cancer developed from cells on the surface of the ovary, an explanation first proposed by British surgeon Thomas Spencer Wells in 18732. This was intuitive enough to gain general acceptance — it seemed to stand to reason that large tumours found on and around the ovaries would have developed from ovarian tissue.
But this model of ovarian cancer was contradicted by clinical reality. In fact, physicians almost never found early-stage cancers, or even precursor lesions, that were confined to the ovary. “The ovarian surface epithelial origin of this disease did not make sense based on the way this disease presents,” Huntsman says.
In 1999, Louis Dubeau — a pathologist at the University of Southern California in Los Angeles — proposed a credible alternative hypothesis. He pointed out that ovarian tumour cells shared characteristics with healthy cells from the fallopian tubes and endometrium, and suggested that the cancer therefore might arise from those tissues3. A few years later, data from people with mutations in ovarian cancer risk genes provided evidence to support that hypothesis. Researchers in the Netherlands, led by gynaecological oncologist Jurgen Piek, reported that fallopian tubes removed during risk-reducing surgery sometimes contained precancerous lesions known as serous tubal intraepithelial carcinoma (STIC)4. (It was common to remove the fallopian tubes along with the ovaries because it was convenient to do so and, in the absence of ovaries, the fallopian tubes were thought to have no function.)
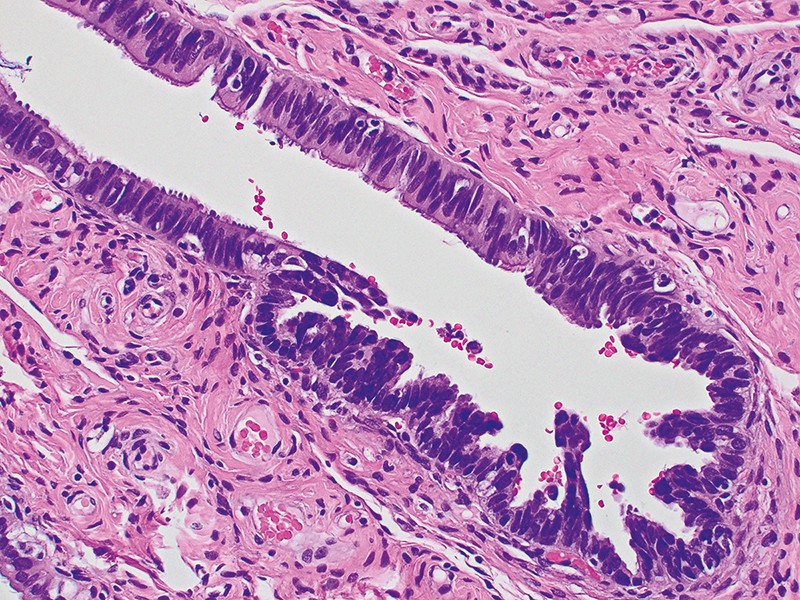
After removing the fallopian tubes during such surgery, pathologists typically gave them only a cursory look, so the significance of these STIC lesions wasn’t clear at first. When Christopher Crum, a pathologist at Brigham and Women’s Hospital in Boston, Massachusetts, decided to look at these structures more systematically in the mid-2000s, he and his team found that STIC lesions were widespread in the fallopian tubes of people with ovarian cancer risk genes, especially at the end nearest the ovary5. “That was really the ‘flip the table’ moment,” says Ronny Drapkin, a gynaecological pathologist at the University of Pennsylvania in Philadelphia, who worked on Crum’s study.
Over the following decade, researchers uncovered increasing evidence linking the fallopian tubes to HGSOC. More pathology studies of risk-gene carriers confirmed the presence of STIC lesions in the fallopian tubes, as well as earlier changes to fallopian tube epithelial cells involving the loss of the tumour-suppressor gene TP536. Mouse model studies showed how fallopian tube epithelial cells could be progressively transformed into HGSOC cells7. Furthermore, studies tracked genetic mutations and gene-expression changes to establish in detail how healthy fallopian tube cells transition into precancerous lesions and then metastatic disease8.
The fallopian tubes are not, however, the origin of all ovarian cancers. Certain rarer kinds of ovarian tumour seem to arise from the ovaries themselves or from other tissues. And STIC lesions can be identified in the fallopian tubes of no more than 60% of people with HGSOC9. Scientists have debated whether this means that the cancer arises elsewhere in some cases or, alternatively, that STIC lesions, which are small, are sometimes simply missed.
Pick and choose
By the early 2010s, the evidence in favour of the fallopian-origin hypothesis of HGSOC was strong enough that physicians, scientists and even some carriers of ovarian cancer risk genes began to consider that it could be effective to remove a person’s fallopian tubes early. A second operation to remove their ovaries would then take place when they were closer to menopause.
Clinical trials have been launched in multiple countries to test the feasibility of this procedure. Researchers approached the question cautiously, given that ovarian cancer is so deadly and that an effective risk-reducing operation was already available. They also sought an unusual degree of input from those affected. In the Netherlands, researchers organized focus groups of people with risk-gene mutations to discuss the aims and intended methods of their study. One of the main things the focus-group participants said was that a trial should not be randomized, says Joanne de Hullu, a gynaecological oncologist at Radboud University in Nijmegen, the Netherlands. “They want to make their own decision.”
The study that came out of those focus groups, known as the TUBA trial, enrolled 577 people with risk-gene mutations and offered them the choice of having their ovaries and fallopian tubes removed at once, or having a sequence of two operations: the first to remove the fallopian tubes and the second, by the age of 40–50, to take out the ovaries10.
Of the participants who have so far undergone surgery, 72% chose to have just their fallopian tubes removed first and 28% chose the conventional surgery. As a group, those who had only their fallopian tubes removed had fewer menopause-related symptoms a year later, the researchers reported in June10.
A similar multi-site trial in the United States, known as WISP, launched in 2016 and is close to wrapping up. The trial, led by Karen Lu, a gynaecologist at MD Anderson Cancer Center, enrolled more than 400 people with risk-gene mutations. About half chose to have the standard risk-reducing surgery and the other half picked the two-stage procedure. Ana Castillo is part of that second group; because she is in her mid-30s, she felt that the choice was straightforward. “I want to take that extra step and be a little bit more careful — and if I can be part of the study, why not,” Castillo says. “I hope that they are able to get some answers for future generations.”
Similar to the TUBA trial, the WISP trial was aimed at investigating whether the two-stage surgery has quality-of-life advantages compared with the standard procedure. Researchers are currently analysing WISP trial data to determine whether delaying the removal of ovaries is associated with greater quality of life, including improving participants’ satisfaction with their sex lives.
Neither the TUBA nor the WISP trial, however, was large enough to properly evaluate the effectiveness of the two protocols in reducing ovarian cancer risk. Both trials had a mechanism to stop the study if an unexpectedly large number of people in the two-stage-surgery group had developed cancer — the threshold for which was set very low, de Hullu notes. This was not triggered in either case. But rigorously evaluating the risk-reducing effectiveness of the two-stage procedure will require larger trials, with a longer follow-up period.
Those trials are now getting under way. Lu and de Hullu are collaborating on an international study known as TUBA-WISP2 that aims to enrol 3,000 people with ovarian cancer risk genes, who will also be given a choice of one- or two-stage surgery. Another trial, called SOROCK, has a similar design but is focused on people who carry a mutated form of the gene BRCA1, who have an especially high risk of ovarian cancer. (People who carry the harmful BRCA2 variant face an 11–17% risk of developing ovarian cancer in their lifetime, compared with about 1.2% of people in the general population. Those with BRCA1 mutations have a 39–44% risk1.) It will probably be another decade before these trials have definitive results, researchers say.
Broader benefits
About 80% of ovarian cancers occur in people who do not carry a known risk gene11. There’s little direct evidence about the origin of ovarian cancer in these average-risk individuals, but many researchers think that the fallopian tubes are likely to play a part in many cases of HGSOC in this group as well. And that opens up a cancer-prevention opportunity for a much broader population, Huntsman says.
A decade ago, physicians in British Columbia in Canada began offering people undergoing surgical sterilization for birth control the option of having their fallopian tubes removed rather than cauterized, as was previously standard. It also became standard practice for surgeons to remove the fallopian tubes as part of hysterectomies. A team of researchers including Huntsman has been tracking rates of ovarian cancer in these groups compared with the general population.
So far, this follow-up has shown that removal of the fallopian tubes doesn’t increase surgical costs or risks, and does not induce premature menopause12. The procedure, known as opportunistic salpingectomy, is now becoming common across Canada and in other countries. Physicians are also talking about extending it to other abdominal surgeries, such as the removal of the appendix or gallbladder.
“After ten years of this, we see the first signal of efficacy,” says Huntsman. These data are not yet published, but Huntsman thinks the study will shift the discussion. “Advising this change in practice will be, I think, considered standard of care,” he says.
Only a minority of people will happen to undergo abdominal surgery at a convenient time for this procedure to be performed. Researchers are therefore also looking for less invasive ways to stave off ovarian cancer. For example, oral contraceptives have long been known to decrease the risk of the disease, as does pregnancy and breastfeeding. In the past, it was assumed that by suppressing ovulation, these factors prevented repeated injury to the surface of the ovary, which could contribute to cancer risk over time. But now, researchers are investigating the interplay between the ovary and the fallopian tubes during ovulation.
The release of hormones and signalling chemicals called cytokines might contribute to the transformation of fallopian tube cells into cancer cells. “The cells are being bombarded with these cytokines and hormones every month,” says Sophia George, a breast- and ovarian-cancer researcher at the University of Miami in Florida. Breaks in the ovarian surface might also give cells that shed from the fallopian tube an opportunity to enter the ovary, take root, and perhaps further transform.
However, although precancerous STIC lesions are thought to form during the reproductive years, ovarian cancer doesn’t usually develop until after menopause. Researchers are therefore investigating how hormonal shifts might drive the process forward. The findings from these studies could lead to oral contraceptives, hormone-replacement therapies or contraceptive devices designed to have a protective effect against ovarian cancer. It might even be possible to develop a drug that could stop early ovarian cancer in its tracks, or prevent the disease altogether. Such advances would be a particular boon in low-income countries, where the prevalence of ovarian cancer is predicted to rise in the coming decades and where risk-reducing surgery might not be widely available, George says.
Window of opportunity
The understanding of the fallopian tube origin of many ovarian cancers helps to explain why these tumours are so deadly, researchers say. Precursor STIC lesions in the fallopian tube are small — perhaps 1–2 millimetres across, and sometimes only a single cell thick — and therefore unlikely to be picked up by medical imaging. And because the fallopian tubes are in the abdominal cavity, abnormal cells that shed from them can easily make their way to the ovaries or elsewhere. This essentially means that most often, by the time ovarian cancer is diagnosed, it has already metastasized.
But the fallopian origin has also prompted fresh ideas for screening and early detection, which has been a particularly difficult problem to solve in ovarian cancer. In a 2017 study that tracked the evolution of HGSOC from normal fallopian tube cells, researchers also calculated a kind of molecular clock for ovarian cancer. They found that it typically takes about seven years for a STIC lesion to evolve into an ovarian tumour, and two more years for the cancer to spread further13.
“It provides a window in which you can potentially detect the cancer and then perform surgery before the cancer becomes a much, much deadlier disease,” says study team member Victor Velculescu, an oncology researcher at the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Velculescu is developing a test that looks for patterns of cell-free DNA fragments in the bloodstream that serve as a fingerprint of cancer14. “If we can detect the cancer early, we could potentially do a resection of the fallopian tube so surgical treatment could be more focused on the fallopian tube rather than the ovaries themselves,” he says.
Other researchers are looking for signs of ovarian cancer in fluid collected from the uterus or as part of cervical smear tests. So far, such approaches are better at detecting advanced than early cancers — but they might yet yield biomarkers that could be detected in the blood earlier in the disease, researchers say. Yet another idea is to use an instrument called a falloposcope to examine the fallopian tubes for precancerous lesions and sample cells — much like a colonoscopy for colon cancer screening. However, some researchers doubt that this would be cost-effective in those at average risk.
None of these possibilities is yet ready for broad clinical roll-out. But as Castillo puts it: “Science goes a long way in a few years, right?”



More News
Author Correction: Bitter taste receptor activation by cholesterol and an intracellular tastant – Nature
Audio long read: How does ChatGPT ‘think’? Psychology and neuroscience crack open AI large language models
Ozempic keeps wowing: trial data show benefits for kidney disease