As Weijian Zong stared at a dazzling grid of green and blue brain cells under the microscope he had built, he felt — for a moment — unstoppable. “I felt that if we could make [this microscope] work, we can do anything we want,” he says. The moment, last March, called for an impromptu laboratory meeting, and a celebration. Zong, an optical engineer at the Kavli Institute for Systems Neuroscience in Trondheim, Norway, changed into a white shirt and tie before showing his latest data to his co-workers. It was, he says, the moment his “toy became a tool”.
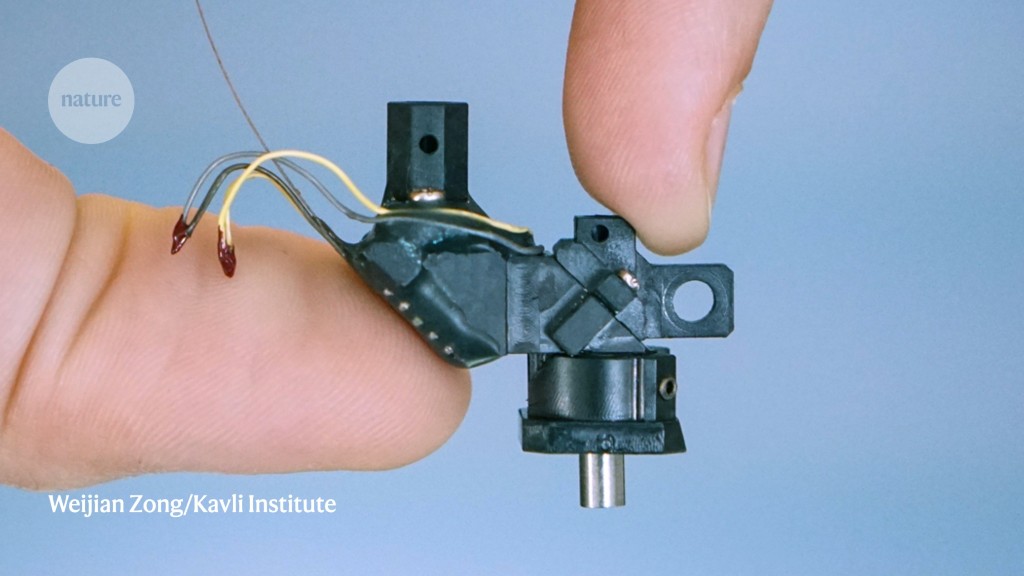
The toy, in this case, is a thumb-sized two-photon fluorescence microscope. It can illuminate and record living tissue at depths that are unattainable with conventional fluorescence microscopes. Weighing just 2.4 grams, the Mini2P can be attached to the head of a mouse and track the activity of hundreds, even thousands, of neurons while the animal runs, climbs and leaps from a platform1. Zong and his colleagues tested the device in the vision, memory and navigation centres of the mouse brain, probing cells as far down as half a millimetre.
With a custom-made lens that can follow the same cells continuously for up to one hour, or multiple times over weeks, the instrument produces much sharper images and can capture similar numbers of cells, if not more, than head-mounted, one-photon ‘miniscopes’, which are the current state-of-the-art for in vivo imaging in freely moving animals. According to Fritjof Helmchen, a physicist-turned-neuroscientist at the University of Zurich in Switzerland, the Mini2P achieves “nearly as good” resolution as a bulky bench-top two-photon system. It is also open-source, with parts lists and instructional videos available on GitHub. In December, 16 researchers will each pay around €5,500 (US$5,370; which includes the cost of parts) to build their own two-photon miniscopes at a three-day workshop hosted by the Kavli Institute in Trondheim.
The Mini2P — for which Zong was awarded this year’s Tycho Jæger Prize by the Physical Society of Norway and the Irma Salo Jæger and Tycho Jægers Foundation — “opens the door to lines of scientific inquiry that were difficult, if not impossible, to initiate”, says Denise Cai, a neuroscientist at the Icahn School of Medicine at Mount Sinai in New York City. And it’s a development that has been years in the making.
Fluorescence microscopy is based on a simple principle: when molecules absorb energy, they become electronically excited; as the molecules ‘relax’, they release light. Most microscopes are designed so that a single photon of light energy is sufficient to induce this reaction. But that can be problematic in thick tissues: as the light passes through the cellular layers, it is absorbed and scattered. Two-photon microscopes circumvent this problem by using multiple, longer-wavelength photons, which can penetrate deeper into tissue (two photons are needed because a single longer-wavelength photon doesn’t have enough energy to excite the molecule). But two-photon systems are bulky and require specialized light sources and lenses. Researchers have worked for more than two decades to shrink the technology into an instrument that is light and compact enough for use in freely behaving animals.
A new way to capture the brain’s electrical symphony
Helmchen was an early pioneer. As a postdoctoral researcher at what was then known as Bell Labs Innovations, a research and development firm in Murray Hill, New Jersey, Helmchen and his colleagues built the first portable two-photon microscope. In 2001, they reported their proof-of-principle device — an ultrafast pulsed laser connected with a 2-metre flexible tether to a 25-gram microscope that could be mounted on the head of a rat2.
The design was the first to demonstrate that a portable two-photon miniscope could record calcium signals (a visual marker of neural activity) from the branched projections, known as dendrites, of individual neurons — but only in anaesthetized, head-restrained rats. The process was also exceedingly cumbersome. The researchers had to manually inject calcium-sensitive dyes into cells one at a time, wait for the cell to light up, then mount the microscope onto the rat’s head and find the cell before attempting to capture a video. The team managed to image just seven neurons over several months, Helmchen says, capturing a single cell in each experiment.
It would take another eight years for a head-mounted two-photon microscope that could image calcium signals in freely moving animals. In 2009, researchers in Germany built a 5.5-g portable system that could track up to 20 neurons at a time. They captured images of the neurons, which were loaded with calcium indicators, in the visual cortices of rats while the animals ran along a semicircular track3. But the design failed to gain much traction, Helmchen says, in part owing to the complexity of the system.
One-photon success
At the time, Zong was a second-year engineering undergraduate working on lasers at Peking University in Beijing. But what he really wanted — his “final dream”, he says — was “to understand nature”. In 2012, he began doctoral studies with biomedical engineer Heping Cheng. Cheng’s lab at Peking University develops fluorescence-microscopy methods for biological research.
By then, single-photon miniscopes were gaining popularity. Lightweight and robust enough for highly active mice, these devices can image thousands of cells at once, allowing researchers to decode entire neural circuits rather than just a smattering of cells. They can also detect GCaMP6, an ultra-sensitive calcium sensor developed after the earliest two-photon prototypes came out. Having been used to track behaviours such as spatial memory, song vocalization and sleep, single-photon miniscopes “have been tremendously successful”, Helmchen says.
Inscopix, a biotechnology company in Mountain View, California, has sold around 1,500 one-photon miniscopes to more than 650 labs, with “well over 220 publications” reporting work using the technology since 2011, according to chief commercial officer Martin Verhoef. The devices cost US$50,000–150,000, depending on the configuration.
Open-source alternatives include a single-photon miniscope from the University of California, Los Angeles (UCLA), which comes with documentation, software and assembly tutorials on GitHub. That costs around $500 if the parts are purchased in bulk, or $1,200 as a do-it-yourself kit. Fully assembled UCLA miniscopes are available for about $2,000 from firms such as LabMaker in Berlin and the Open Ephys Production Site in Lisbon.
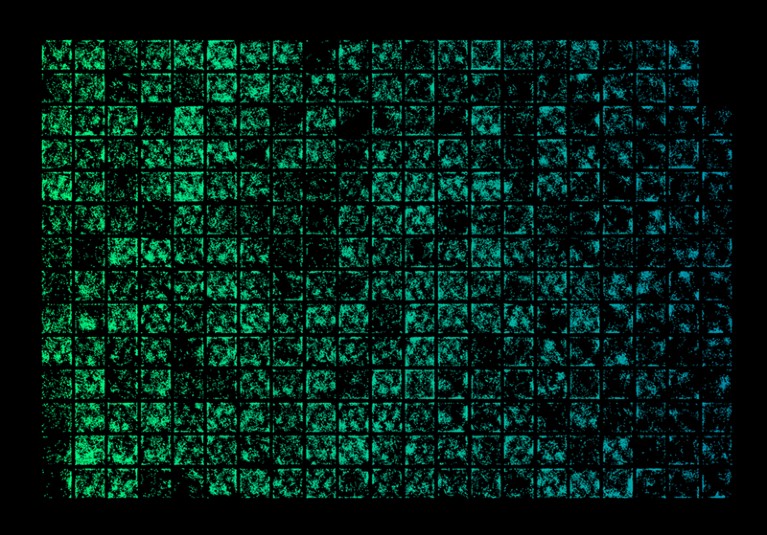
The Mini2P device can record 300 grid cells in a single mouse.Credit: W. Zong et al./Cell (CC BY 4.0)
Around 500 labs worldwide have used the UCLA miniscope (now in its fourth iteration) since the device was first built and shared about a decade ago, says neuroscientist Peyman Golshani, whose lab at UCLA helped to develop it. Researchers have used it to study neurons that encode memories across time, for example.
Despite their capabilities, one-photon miniscopes generally cannot image deeper than a few hundred micrometres, and the resulting fluorescence is out of focus and can blur images. That’s not typically a problem in brain areas such as the hippocampus, where only small subsets of cells are firing, so the cells are sparse enough to identify in cloudy images, says Edvard Moser, who, along with May-Britt Moser, heads the Kavli Institute for Systems Neuroscience.
The resolution does, however, pose a problem in the Moser lab. Researchers there study grid cells — specialized neurons that store information about location, distance and direction. Their discovery of these cells earned the Mosers a share of the 2014 Nobel Prize in Physiology or Medicine. But single-photon microscopy is “not sufficient” to image grid cells, says May-Britt Moser. “You have to have the resolution of two photons.”
Miniscope collaborations
Enter Zong. In 2015, he was part of a team, led by Cheng, that received a grant to build a device combining the lightweight features of one-photon miniscopes with the subcellular resolution of large bench-top two-photon systems.
In 2017, they described a fast, high-resolution, miniature two-photon microscope that produced one-hour recordings of neural activity from tiny nerve-cell protrusions called dendritic spines4. The microscope’s resolution nearly matched that of large, bench-top two-photon systems.
The microscope makers putting ever-larger biological samples under the spotlight
The researchers used a customized photonic crystal fibre — similar to those used in telecommunications to transmit information with fast, short pulses of light. The fibre delivered laser pulses at 920 nanometres — a wavelength that excites GCaMP6. (Earlier two-photon-miniscope designs transmitted 800-nm light and were limited to less-sensitive calcium sensors.) The team added a miniature objective lens with higher resolving power than earlier designs and used a custom scanning mirror to boost imaging speed. The resulting device could image brain activity down to the level of neural synapses — the connections between neurons — in active mice.
According to Edvard Moser, this miniscope was “already a revolution”. It showed that “you can actually image cells at high resolution and with high stability in animals that move around”, he says. “People were shocked” — not least of all himself.
Edvard Moser first met Zong in October 2017 at a conference outside Beijing. Excited about the potential of the two-photon miniscope, Moser had scheduled a visit to Cheng’s lab. Zong was sent to pick him up and drive him there.
Chatting during the two-hour journey, Moser learnt more about the two-photon miniscope. “The resolution was superb, and the weight of that microscope was not bad at all,” he says. But with a small field of view (130 × 130 micrometres), the miniscope could image only a few dozen cells — too few for what Moser and his colleagues needed. “You can’t see the [neural firing] patterns when you have so few cells,” he says. By comparison, a commercial bench-top two-photon microscope can image more than one square millimetre.
Still, the first-generation device represented a huge advance over previous two-photon head-mounted designs, Moser says. It just needed a “little extra to be useful”.
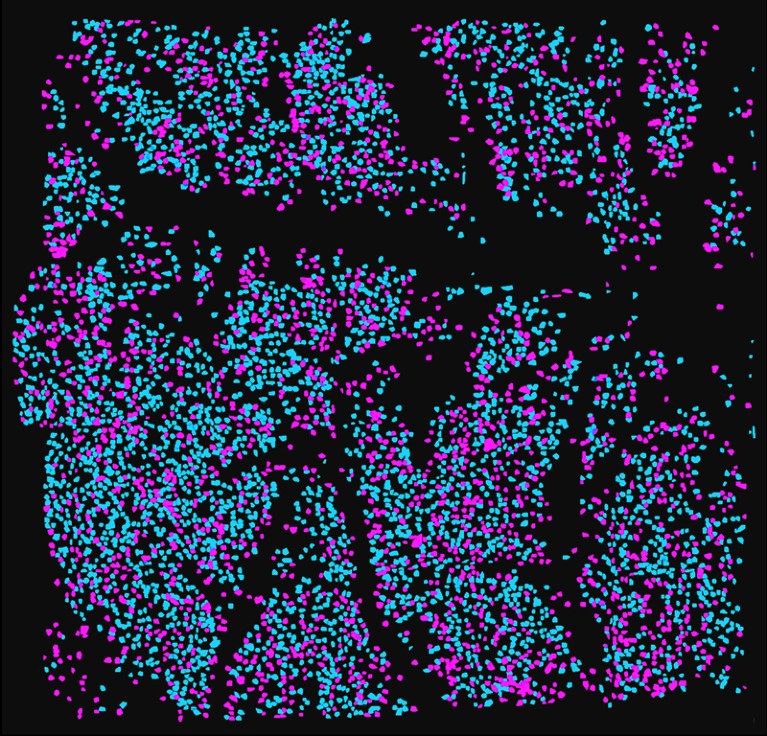
Composite image of 25 stitched-together fields of view, showing about 10,000 cells at 2 depths (blue, 100 μm; magenta, 140 μm).Credit: W. Zong et al./Cell (CC BY 4.0)
Six months later, Zong moved to Norway and joined the Mosers at the Kavli Institute, where he could continue developing the two-photon miniscope with input from biologists who were eager to use it. The Moser lab had spent more than two decades investigating spatial navigation — animals’ ability to track changes in position and orientation during movement. This complex behaviour is regulated by networks of grid cells, which fire at regular intervals as animals navigate open space. But understanding how the activity of these specialized neurons is coordinated requires careful scrutiny in freely moving animals.
Last year, Zong and his colleagues in China described5 a second version of their miniscope, featuring an expanded field of view and a larger working distance that allows researchers to scan multiple planes and image a 420 × 420 × 180 micrometre volume. This second-generation instrument was robust enough to record neuronal activity from the same brain area for weeks.
The microscope makers
But the enhancements came with trade-offs. The microscope was heavier and the cable more rigid, which constrained how freely mice could move, says May-Britt Moser. Plus, the design included an electrically tunable lens that would heat up as researchers moved it up and down. That heating caused optical drift, which changed the position of cells in the field of view. “For a neuroscientist, that’s a disaster, because you can’t then monitor activity in the same cells over time,” says Edvard Moser.
In Trondheim, Zong discovered an alternative tunable lens called the TLens, made by optics technology company poLight in Skoppum, Norway.
Designed for mobile-phone and smartwatch cameras, the TLens seemed ideal for the two-photon miniscope, Zong says. It is tiny and fast, and thanks to a fundamentally different mechanism for tuning the lens’s optical power, has better thermal stability. It needed just two adjustments. First, Zong worked with firm Sunlight Technology in Fuzhou, China, to change the optical coating of the TLens to make it compatible with two-photon laser wavelengths. Second, scientists at poLight made a stack of four of the modified lenses to extend the optical range and incorporated it into the new miniscope1.
The TLens array allows the Mini2P to image on multiple planes, essentially producing a picture of a volume of tissue. That boosts the number of neurons that researchers can record into the thousands — similar to the number recorded by single-photon miniscopes. This was “a major advance”, says Helmchen. Importantly, the miniature lens, along with a more flexible cable, allowed mice to move without restraint — a crucial improvement over the bulkier 2021 set-up.
“The [Mini2P] paper nicely shows that the mouse behaviour with this new version of the microscope is similar to that of a mouse that doesn’t carry a microscope,” says Yaniv Ziv, a neuroscientist at the Weizmann Institute of Science in Rehovot, Israel. “It is also quite impressive and important for experiments in more naturalistic settings that the field of view remains stable during more vigorous behaviour of the mouse.”
NatureTech hub
Ziv is one of the 16 researchers who will build their own Mini2P at the December workshop at the Kavli Institute. His lab uses one-photon miniscopes to study how long-term memories are encoded in the hippocampus, but the researchers cannot distinguish between cells in deep and superficial layers. The Mini2P, he says, might succeed where the one-photon miniscope could not. Another workshop participant, neuroscientist Yi Gu at the US National Institutes of Health (NIH) in Bethesda, Maryland, studies how the brain encodes spatial information, using a bench-top two-photon microscope to record cells while mice with their heads fixed in place navigate a virtual linear track. But this experimental set-up doesn’t allow recording of cells such as head-direction neurons, which sense changes in position and movement of the head. Gu hopes that the Mini2P will allow her to perform these experiments in freely moving mice while they navigate real environments.
A complex build
Other researchers are also on the hunt for a better miniature two-photon microscope. In August, several months after the Kavli team reported the Mini2P, an independent team led by Golshani received a $4-million grant through the NIH’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative to develop two new two-photon miniscopes: a mouse scope with an 800 × 800 µm field of view — nearly twice as large as the Mini2P’s and comparable with a bench-top two-photon set up; and a device that will allow researchers to image multiple brain layers at the same time in rats and larger animals. For his part, Zong says, he and his colleagues “will definitely continue to develop” the Mini2P, “to make it wider, faster and deeper”.
What might be harder to achieve is community uptake. It’s particularly challenging for developers of open-source tools to make their systems stable as well as easy to build and repair by people who are not microscopy specialists, says Ziv, and that’s especially true of two-photon microscopy. The method requires lasers that produce ultrafast (on the order of one millionth of a nanosecond), high-power light pulses and cost as much as $200,000. “That price tag is prohibitive for many research groups,” Cai says. Two-photon systems are also much more complex than one-photon set ups, so building and using the Mini2P requires considerable technical expertise.
But in Trondheim, development of the Mini2P continues. Three iterations in, “we are just beginning”, Zong says. “Every technique is already old when it is published.”






More News
Who will make AlphaFold3 open source? Scientists race to crack AI model
These crows have counting skills previously only seen in people
Seed-stashing chickadees overturn ideas about location memory