Credit: Oli Winward
All four of Danny Benjamin’s sons, aged 14 to 20, took part in clinical trials of COVID-19 vaccines. Two of them were among the first children in the United States to be vaccinated. “They’re super pumped about having done that,” says Benjamin, a paediatrician at Duke University in Durham, North Carolina. “They’ve posted it on their Instagram accounts.”
But Benjamin, who also chairs the National Institute of Child Health and Human Development’s Pediatric Trials Network, thinks that it took too long for the COVID-19 vaccines to be tested in children. He thinks that all the clinical trials on children should have been completed by May 2021, by which point some countries had already administered millions of doses to their adult populations. Instead, trials in children didn’t even start until March that year, and some still haven’t concluded. “We should have had all the children’s studies done by May if we’d been ethical and honourable about how we developed COVID vaccines, rather than have children suffer for another year,” he says.
Part of Nature Outlook: Children‘s health
The shortage of paediatric clinical data is not limited to COVID-19. Although the number of trials in kids has increased over the years, lack of data is still delaying paediatric labelling of drugs and leaving physicians with little information about whether the drugs are effective or safe in children.
An analysis1 of around 11,000 prescriptions for children at Swedish hospitals in 2008 found that nearly half were off-label, meaning the medicines were not yet approved for paediatric use by the European Medicines Agency (EMA). The youngest children in the sample were prescribed off-label medicines at the highest rate. “We don’t have a better alternative,” says Jenny Kindblom, a paediatric clinical pharmacologist at the University of Gothenburg in Sweden.
The problem with that approach, says David Ziring, a paediatric gastroenterologist at Cedars-Sinai in Los Angeles, California, is that children are not small adults. “The drugs act on children’s bodies very differently,” he says. Without trials to determine how a drug should be used in children, “we’re left using either drugs that are 30 to 40 years old and less effective, or trying to use the most recently approved drugs and trying to justify to insurance companies that, despite its lack of an FDA [US Food and Drug Administration] label for paediatrics, we feel that it would be safe and effective,” he says.
It usually takes at least seven years for adult-approved drugs to be authorized for use in children, Ziring says. For years, he has been advocating for drugs to be approved for paediatric use faster, but to little avail. “We’ve made very little progress,” he says. “The community of paediatric sub-specialists that I belong to has become very frustrated.”
For a long time, there was little incentive for pharmaceutical companies to pursue paediatric labelling with any urgency, Ziring says. Paediatric trials are generally slower and more expensive than adult ones because it is harder to recruit participants, the ethical bar is higher and there is less money to be made.
But in 2002, the US Congress passed the Best Pharmaceuticals for Children Act, under which further marketing exclusivity is given as an incentive to firms that voluntarily conduct paediatric trials on drugs identified as a priority by the US Food and Drug Administration. Similarly, in 2007, the European Union brought in regulations in a bid to improve access to medicines for children, increase transparency around product information and boost the amount of paediatric research.
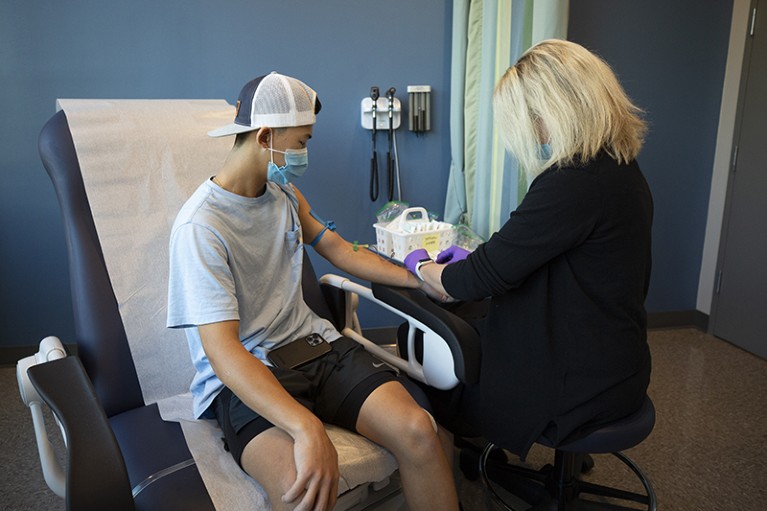
In October, Danny Benjamin’s son, Scotty, participated in a Pfizer COVID-19 vaccine trial at Duke Health in Durham, North Carolina.Credit: Duke Health Photography
Although clinical trials in children remain few, data suggest that they are on the rise. A 2021 study2 found that between 2008 and 2010, just over 7,000 paediatric trials were registered on the US clinical trial registry ClinicalTrials.gov. But between 2017 and 2019, this figure had increased to around 11,700. However, most of these trials were small-scale, single-site, and not funded by the US National Institutes of Health or by industry. Ziring thinks it will take new legislation for pharmaceutical companies and the FDA to work towards earlier approval of paediatric medicines. “There’s only so much that paediatricians can do.”
But there are some signs of progress. In 2020, the eczema drug dupilumab became the first biologic medicine to be approved for children aged six and up, just three years after it won approval for use in adults. And in June this year the therapy, made by French multinational pharmaceutical company Sanofi and the US biotechnology firm Regeneron, was extended to children as young as six months. “Somehow they were able to most efficiently enrol a very large cohort of children,” Ziring says. “Hats off to them.” The approval shows that early paediatric labelling of drugs is possible, he adds. “If there’s a commitment from pharma and they work closely with the FDA,” he says, “that is very doable.”
Obstacles to success
Lack of incentives for drug makers to test treatments in children are not the only reason that paediatric trials are few and far between. Even for motivated researchers, getting a paediatric trial off the ground can be tough.
First, it’s harder to obtain ethical approval for children’s trials. For a trial to be considered ethical, the risk to the child from participating has to be no greater than the risks that they experience in their everyday life, says Patrina Caldwell, an academic paediatrician at the University of Sydney in Australia. That has led some companies to move their trials to low-income countries, where children are considered to be exposed to greater risk on a daily basis and the bar is therefore set lower. “Sometimes drug companies have gone to developing countries to jump through the ethical hoops,” Caldwell says, but she thinks it is unethical to treat the lives of poorer children as less valuable. Besides, she adds, trials in low-income countries are often said to be reviewed less rigorously and conducted less robustly, so results might not be accepted by regulators in high-income nations.
The higher ethical bar for children’s trials means that even simple tests and procedures to monitor progress can be more complicated, says Caldwell. “In an adult trial, you can just do a blood test pretty easily, but in a paediatric trial you’d have to justify why you need to do it,” she says, because the tests are considered more invasive and distressing for children.
Even if the trial is approved, it can be difficult to find volunteers. Childhood diseases are rarer than those in adults, so there are fewer people to choose from. To recruit enough children, trials often have to be run concurrently in several locations. “There are complexities to doing multicentre studies because it involves different regulatory systems, research centres, different rules and regulations, and different governments,” Caldwell says. “That in itself is a nightmare.”
Offering monetary incentives to encourage participation is a common way of boosting trial numbers in adults, but it is controversial in children’s trials because the process could be prone to abuse in cases in which parents enrol their children solely for material gain, Caldwell says. In many countries — including Australia, for example — ethics committees do not condone paying children to participate in trials.
Another problem is that parents are often worried about the possible long-term side effects that could result from experimental drugs, Caldwell says. She recalls parents asking: “What if one day this drug is proven to cause cancer, and when they’re 60 they have cancer and they blame me for something I let them be part of when they were younger?”
More from Nature Outlooks
Caldwell, however, says she doesn’t usually have trouble convincing parents once she explains that trials in children generally don’t have a placebo arm, unlike those in adults. Instead, trials in kids typically use what is called clinical equipoise — testing two treatments to determine which is more effective. “For a trial to be ethical in a child, there needs to be equipoise,” Caldwell says. Explaining this conveys uncertainty while giving parents the autonomy they need to make their decision, she says. When a child has a life-threatening condition, parents are more at ease knowing that their child will receive at least one potentially effective treatment rather than a placebo.
Although there is an overall lack of child participants for studies, once the parents of kids who can take part are convinced, then children do tend to enrol at higher rates than in adult trials. Caldwell notes that the survival rate in children with leukaemia has gone up significantly3. The health outcomes in adults aren’t anywhere near as high. “It basically shows the power of trials,” she says.
What’s more, research suggests that participants in trials — regardless of which study arm they are in — have better health outcomes than those who are not involved in any trials4. The concept, known as the Hawthorne Effect, suggests that people behave differently when they know they are being watched, so participants are more likely to lead a generally healthy lifestyle than are non-participants.
Those running the trial need to carefully explain both the risks and the benefits, and must themselves be sure that participation is the right thing for the individual. “The people who are recruiting for trials have to really believe in the trial,” Caldwell says.
Ultimately, she thinks that governments might need to intervene directly to fund more paediatric research. “It’s not fair that children don’t have research data for themselves because people won’t do it if it’s not making money.”







More News
Finding millennia-old ‘monumental’ corals could unlock secrets of climate resilience
Argentina’s pioneering nuclear research threatened by huge budget cuts
The dream of electronic newspapers becomes a reality — in 1974