ONA Therapeutics is a spin-off from the Institute for Research in Biomedicine, Barcelona, Spain, and one of the final eight for The Spinoff Prize 2021.
Salvador Aznar Benitah didn’t set out to show how the metabolic rewiring of cancer cells is crucial to their spread. The molecular biologist at the Institute for Research in Biomedicine in Barcelona, Spain, simply wanted to know what set metastasis-initiating cells apart from other kinds of stem cell with tumour-propagating potential.
Working with tumours of the tongue and throat, he and his colleagues isolated a population of what they thought might be metastasis-initiating cells. A comprehensive genetic analysis revealed increased activity in dozens of genes known to be involved in tumour dissemination. That meant they were on the right track.
But, unexpectedly, the researchers noticed that 17 other genes, each linked in some way to fatty-acid metabolism, also had elevated expression levels. “That of course intrigued us a lot,” Aznar Benitah recalls.
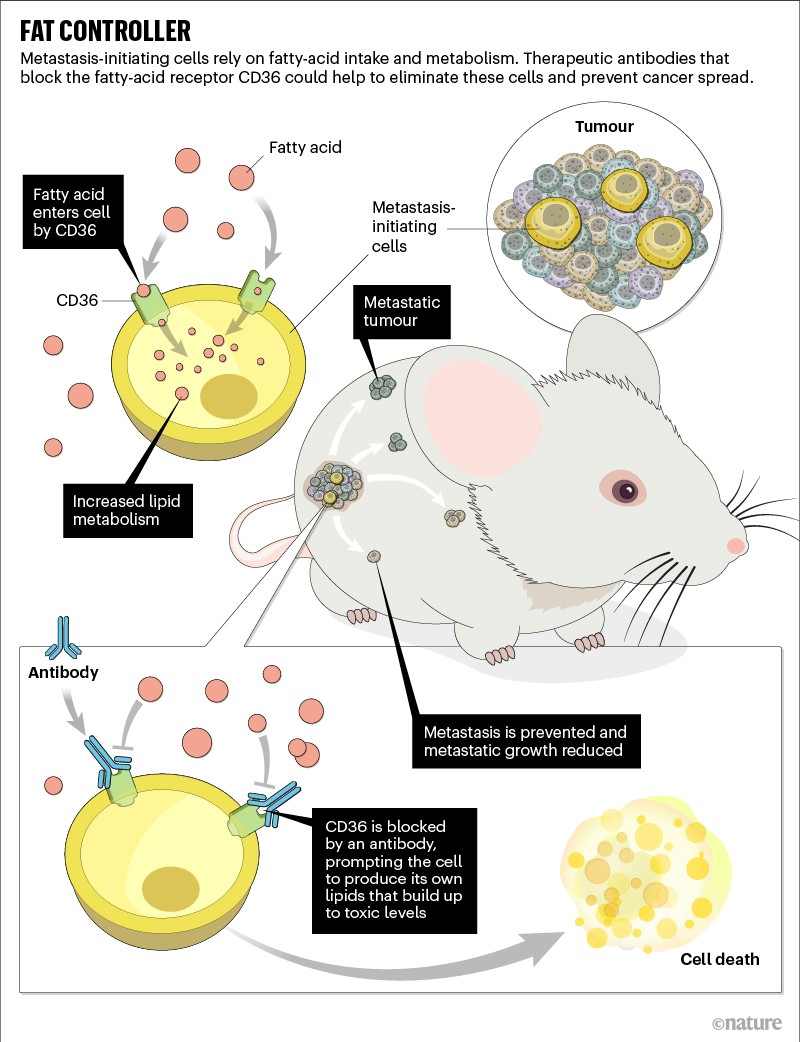
His team probed a little deeper, and in 2016 it showed how one of the active fat-metabolism genes encoded a cell-surface receptor called CD36 that proved indispensable for cancer metastasis1.
In mice with localized tumours, blocking CD36 with a therapeutic antibody completely prevented cancer spread without having any effect on the original tumour (see ‘Fat controller’). What’s more, in mice in which the cancers had already broken away, the therapy reduced the size of metastases by as much as 90%.
After looking at medical records, Aznar Benitah thought that targeting CD36 might work in people, too. Tumour biopsies from people with various types of cancer revealed a strong link between the presence of CD36-expressing cells, metastatic spread and poor survival outcomes. The finding dovetailed with an article that independently described2 the same clinical association.
ONA Therapeutics in Barcelona was formed in 2019 to take the research further. Within a year, the company went from a fledgling university spin-off to one of the most well-funded start-ups in Spain. In June 2020, the company raised €30 million (US$35 million) in venture capital financing to advance a humanized version of the CD36-targeted antibody to early testing in people.
“It’s a really promising strategy,” says Yekaterina Zaytseva, a cancer-metabolism researcher at the University of Kentucky College of Medicine in Lexington. “If you inhibit this lipid metabolism, cancer cells won’t be able to colonize secondary sites as easily,” says Zaytseva, who has studied CD36, but is not involved in ONA Therapeutics. But, she adds, “we need the proof that this targeting can be translated into clinical trials”.
The story on fatty-acid receptors
ONA Therapeutics is now working towards that goal. So far, the company’s eight full-time scientists have validated Aznar Benitah’s earlier work in additional tumour models, further detailing how fatty-acid uptake by CD36 triggers a pro-metastatic signalling cascade. They have also optimized the antibody drug for use in people and continue to study its dynamics.
“CD36 is not an easy target to work with, and it’s quite a new target in this field,” says the company’s co-founder and chief executive, Valerie Vanhooren. “So, we really have to understand the full pharmacology more — but we are making great progress.”
Part of that pharmacology might involve drug engagement with more than just the cancer cells themselves. That’s because some types of immune cell found in and around tumours respond to CD36-mediated signalling in ways that sustain cancer progression.
Research by different teams, one led by cancer immunologist Ping-Chih Ho at the University of Lausanne in Switzerland, has shown that regulatory T cells (which generally suppress immune responses) rely on CD36 to survive3, whereas killer T cells (which normally attack tumours) will undergo a form of cell suicide when fatty acids in the tumour microenvironment trigger CD36 signalling4. The tumour-supporting metabolic adaptations of each type of T cell might be different, but the impact of targeting CD36 with an antibody is the same: the drug alters T-cell function in ways that enhance overall anti-tumour immunity.
“Targeting CD36 can be quite an amazing magic bullet to induce multiple beneficial effects for cancer treatment,” says Ho, who has also filed patents related to the research and is exploring the possibility of forming his own company centred around CD36 blockade.
“On the one hand, you can target this pathway to block cancer-cell metastasis” — as Aznar Benitah’s team has shown — “and on the other hand, you also have the chance to boost host immune responses by targeting T cells,” Ho says.
Vanhooren welcomes the complementary findings of researchers such as Ho. She is confident about the direct effects of CD36 blockade on metastasis-initiating cells — her team and Aznar Benitah’s lab group have both demonstrated the effects in mice that lack an immune system and therefore do not have T cells capable of responding to the therapy. And, she says, this remains the main therapeutic focus of the company.
To her, any extra immune-driven anti-cancer effects are an added bonus. “It’s only beneficial,” Vanhooren says. Clinical trials of the therapy could start in the next two years.








More News
Argentina’s pioneering nuclear research threatened by huge budget cuts
The dream of electronic newspapers becomes a reality — in 1974
Japan can embrace open science — but flexible approaches are key