When neurologist Reisa Sperling stepped up to receive her lifetime achievement award at an international Alzheimer’s conference last December, she was more excited about the future than about celebrating the past.
What thrilled Sperling, who won the award for her work on clinical trials of Alzheimer’s treatments, was a sense of hope, which has been conspicuously missing from research into the disease for many years. Most other attendees felt the same.
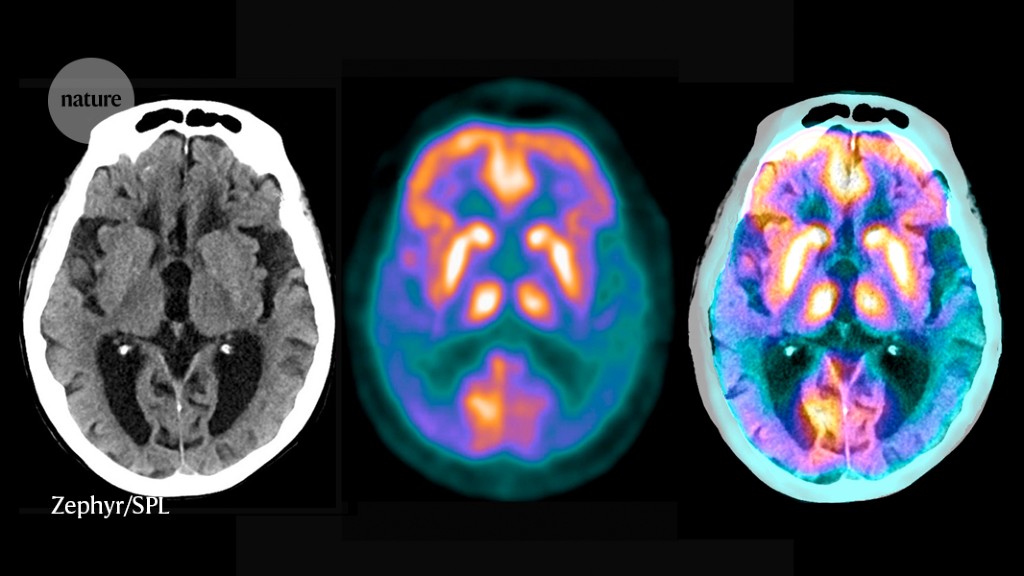
Just a few months before the meeting, researchers had announced that an antibody drug called lecanemab clearly lowered the amount of amyloid protein plaques — a tell-tale sign of the disease — in the brains of participants in a clinical trial, and slowed their cognitive decline.
Sperling, who runs a laboratory at Harvard Medical School in Boston, Massachusetts, was buoyant as she gripped the microphone tightly. After spending more than 30 frustrating years in Alzheimer’s research, she said, there was finally proof that she and her colleagues were on the right track. “But still, it isn’t enough,” she said.
In the trial, treatment led to a 25% slowing of decline, enough to give participants a few extra months of independent living1. “But actually conquering a destructive disease that affects tens of millions of people worldwide is a different story,” she says.
What’s more, lecanemab, marketed in the United States as Leqembi, makes for a tough treatment regime. It has to be infused through a vein by a nursing professional. And because the drug can cause potentially life-threatening brain swelling and bleeds, people taking it have to be monitored regularly.
Could drugs prevent Alzheimer’s? These trials aim to find out
A similar antibody, aducanumab, was approved by the US Food and Drug Administration in 2021, but the decision generated controversy because the drug’s clinical trial had not shown unambiguous benefit.
Despite these wrinkles, the lecanemab results consolidated the hope that Alzheimer’s might eventually be preventable, if treatment is given early enough. The success also raised another possibility: this and future drugs could be used in combination to tackle different stages of the disease, which are often governed by different molecules. Few expect a single therapy to be the answer. But combination-therapy trials are expensive and complicated, because each drug has to be tested both on its own and with its partner. Pharmaceutical companies can be wary of hitching their product to another, in case the combination fails and casts a shadow over their drug.
The mood of greater confidence among those in the field is not only down to the success of anti-amyloid antibodies. The cupboard is stacked with potential new therapies — and abandoned drugs that are now being dusted off.
Promising combinations
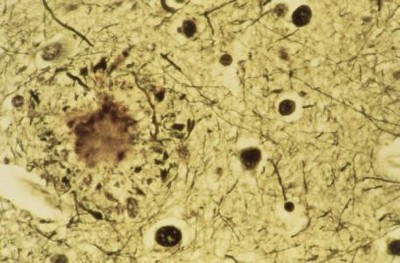
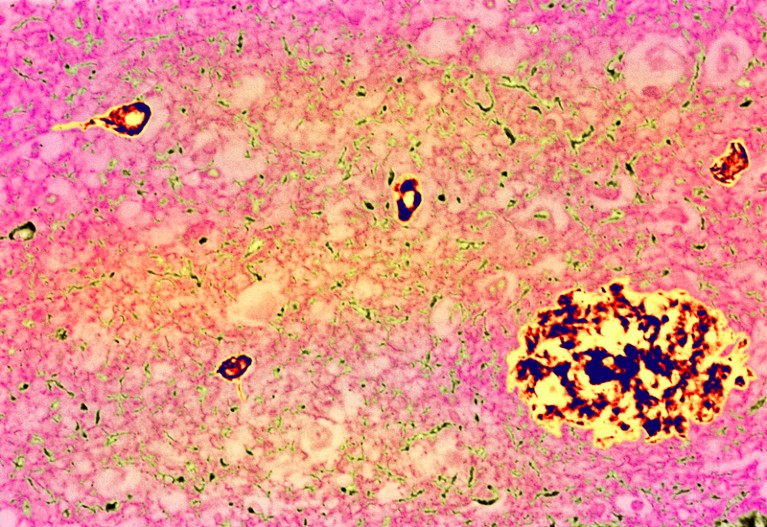
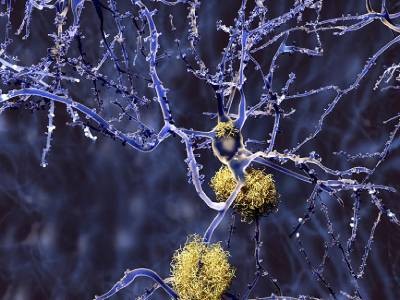
Alzheimer’s disease has a long, silent beginning. First, plaques — clumps of sticky amyloid-β proteins — start to gather in the brain. They quickly become surrounded by immune cells called microglia that try, but ultimately fail, to nibble them away. The plaques grow in size and number but go unnoticed for years, even decades, until they cause another protein — called tau — to accumulate to toxic levels and spread around the brain in tangles. Scientists are still working out exactly how this chain of events occurs, but cognitive symptoms begin to emerge only when it is well under way. The severity of the symptoms correlates with the extent of the tau tangles.
Individual therapies directed at tau have so far not performed well in trials2. But scientists think that tau-busting drugs might work better when paired with anti-amyloid therapy.
“We know that amyloid somehow drives the accumulation of pathological tau, which then spreads through the brain like wildfire,” says Randall Bateman, a neurologist at Washington University in St. Louis, Missouri. “So we think it makes sense to remove the substance that is fuelling the flames, while we try to put out the fire of tau tangles.”
Randall Bateman (left) and colleagues will run a trial of anti-tau and anti-amyloid antibodies.Credit: Matt Miller/Washington University School of Medicine
Bateman and his colleagues began planning such a trial in 2015, but it became feasible only recently, after the first hints emerged that amyloid therapies might prove effective. Last year, they launched an international trial known as Tau NexGen. They are recruiting 168 participants, all of whom are likely to develop Alzheimer’s disease at a young age — typically in their 30s, 40s or 50s — because they have a mutation in a gene that leads them to overproduce amyloid-β.
Participants are being divided into two groups, according to whether they already have symptoms of dementia or are expected to develop symptoms in the next ten years (these people usually become symptomatic at around the same age as the parent from whom they inherited the mutation).
All participants will be given lecanemab and a tau-reducing antibody, but in different orders. Those without symptoms will receive the anti-tau antibody E2814 for a year, then have lecanemab added; the symptomatic group will receive lecanemab for six months and then have E2814 added. The researchers running the trial hope they can use this set-up to learn about optimal treatment combinations.
More anti-tau drugs will eventually be included in the study, and the first results of the trial are expected after 2027.
Tau NexGen is the first, and so far only, ongoing clinical trial of a combination therapy for the condition. A similar, US-based, trial3 is being planned for sporadic, late-onset Alzheimer’s disease, which affects older people and accounts for the overwhelming majority of cases. The US National Institutes of Health (NIH) is expected to decide in the next few months whether it will co-fund this effort, called the ATP trial, as a public–private partnership with drug companies. If it does, recruitment could start next year.
Many pharmaceutical and biotechnology companies are developing anti-tau therapies, some as antibodies, others using other small molecules or newer genetic approaches to block the production of pathological forms of tau. ATP trial co-leader Adam Boxer, a neurologist at the University of California, San Francisco, says that several of these companies have formally expressed interest in participating.
Alzheimer’s drug slows mental decline in trial — but is it a breakthrough?
Like Tau NexGen, this will be a type of prevention trial. Participants will have few or no detectable symptoms, but will have evidence from blood tests and scans that their brains contain plaques and early signs of tau tangles. There will be about 900 participants in 6 arms, who will receive one of two tau therapies either alone or in combination with lecanemab, lecanemab alone, or a placebo.
The research teams hope that anti-tau treatment will boost lecanemab’s modest benefits — and that, in a virtuous circle, by reducing plaque burden, lecanemab will create better conditions for anti-tau therapy to work.
Key to the trials is a range of sensitive new biomarkers — measurements of the brain or blood that give a read-out of disease status. Brain scans monitor the presence and severity of amyloid plaques and tau tangles; tests on blood or cerebrospinal fluid measure many other molecules in the chain of pathology, such as different forms of amyloid and tau. Researchers expect that the extensive molecular and clinical data that they produce will help to uncover more about the mechanisms of Alzheimer’s. “Evidence so far points to tau as the initiator of Alzheimer’s symptoms, disabilities and eventually death,” says Boxer. “But this hypothesis needs to be tested in humans.”
Trials of combination therapies have some drawbacks: they are complex and costly to manage. Boxer estimates that the ATP trial will run to many hundreds of millions of dollars, despite the new biomarkers, which make trials a lot more efficient.
Antibodies themselves make for expensive therapies. Lecanemab will be marketed at US$26,500 for a year’s treatment. Aducanumab (sold as Aduhelm in the United States) was originally priced at $56,000 for a year’s treatment, but the makers halved the price after public outcry.
The drugs are also inconvenient for patients because they have to be infused every few weeks. Data from clinical trials suggest that life-long therapy will be required to keep Alzheimer’s at bay, says neurologist Paul Aisen, at the University of Southern California in San Diego, who is a leader of the US Alzheimer’s Clinical Trials Consortium. “The disease appears to rebound when infusions stop,” he says. Because long-term antibody treatment is impractical, he says, “we think it might make sense to maintain a low amyloid state with an oral drug that blocks generation of the peptide” once the antibodies have removed the plaques.
Such compounds do exist. Beginning around 2010, researchers trialled a suite of oral drugs that aimed to reduce amyloid in the brain by tuning down the activities of one of two enzymes, β-secretase and γ-secretase, which are key to its production. But clinical trials of these drugs all flopped4, and interest in them dried up — until they got a second chance.
Other ingredients
In 2018, a group of drug companies agreed to do something unusual in their normally secretive industry. They decided to share confidential clinical data from six failed trials with each other and a select group of specialists who had been brought together by the Alzheimer’s Association, a lobby group for patients’ rights based in Chicago, Illinois.
The association wanted to learn as much as possible from the disastrous clinical trials, each of which tested a different inhibitor of the β-secretase enzyme. None of the drugs had shown benefit — and worse, many had toxic side effects, including, in some cases, worsened cognition. Rather than let the trial data gather dust behind closed doors, the association wanted them discussed in detail. The goal, says the association’s chief scientific officer, Maria Carrillo, was “to help the field understand more about the disease-related biologies targeted by these investigational drugs”.
Classical features of Alzheimer’s: a plaque (large clump) and tau tangles (small clumps).Credit: Simon Fraser/Science Photo Library
The panel’s review paper, published in 2021, suggests that the disease might have been too advanced in trial participants for this class of drug to improve symptoms, and that lower doses could avoid side effects5.
Aisen thinks it should be possible to use these drugs at low doses to stop plaques coming back — once existing plaques have been cleared with antibodies.
A batch of clinical trials targeting γ-secretase also failed. But rather than abandon the target, researchers have been working on a more nuanced approach. Instead of completely blocking the enzyme — a blunt tool that contributes to the toxic side effects observed in trials — they hope to change its behaviour.
One such modulator, a compound developed in an academic collaboration6, will be tested in an early clinical trial this year. The drug, which can be taken orally, causes the enzyme to chop amyloid up into shorter proteins, which are non-toxic and could even be protective. The trial will be sponsored by a new start-up company, Acta Pharmaceuticals in Boston, Massachusetts, and funded by the NIH.
Most drugs being considered for combination trials target amyloid or tau. But there are earlier-stage approaches that seek to improve the brain’s natural immune-defence mechanisms against Alzheimer’s. Once again, researchers have learnt a lot from families with genetic mutations that predispose them to Alzheimer’s.
The mutation in question is in a gene called TREM2, which makes a molecule that sits on the surface of the brain’s immune-system warriors, microglia. “Tuning up microglia could make them more efficient in clearing plaques or prevent amyloid pathology from spreading,” says neuroscientist Christian Haass at the Ludwig Maximilian University of Munich in Germany, “particularly if the plaque load is first reduced with anti-amyloid therapy.” He is planning experiments in mice to test how an antibody that binds TREM2 and activates microglia might work if it is taken alongside anti-amyloid therapy. A similar antibody is in an early-phase clinical trial as a solo treatment.
Vaccines, genes and plasma
More approaches to stemming Alzheimer’s are making their way into clinical trials. Researchers aim to deliver useful molecules to the brain — through vaccines, viral vectors or blood transfusions.
Like the enzyme drugs, vaccines are being reinvented, long after the first clinical trial of an anti-amyloid vaccine was halted in 2002, when brain inflammation was observed in some participants.
FDA approves Alzheimer’s drug lecanemab amid safety concerns
There are now several anti-tau and anti-amyloid vaccines in preparation or in early-phase clinical trials. They comprise snippets of the tau or amyloid-β proteins, chosen and packaged to avoid serious inflammatory responses. They are designed to stimulate the brain’s immune system to recognize and destroy the full versions of the proteins, and were intended mainly to prevent disease or to slow the progression of early disease. Scientists are even trying to develop vaccines to attack both tau and amyloid-β.
Other researchers are betting on gene therapy to conquer forms of Alzheimer’s caused by gene mutations.
Distinct forms of the APOE gene, which encodes a protein involved in fat metabolism, affect the risk of Alzheimer’s disease in different ways. The APOE4 allele is linked to increased risk, whereas the APOE2 allele decreases it. For its open-label trial, in which participants all received the therapy, Lexeo Therapeutics, based in New York City, recruited 15 volunteers with mild Alzheimer’s symptoms who have 2 copies of the APOE4 gene. They wanted to test whether delivering the APOE2 gene variant would mitigate the harmful effect of the higher-risk version. So they attached the gene to a viral vector, which they then injected directly into the volunteers’ spinal fluid.
The gene graft seems to have been successful. Lexeo reported last year that the APOE2 gene has been detected in the spinal fluid of some participants up to a year after injection, that no serious side effects have been observed so far and that the participants’ tau levels have gone down. It’s too early to assess whether the disease progression has slowed as a result, but participants will be monitored until 2028.
Gene therapy won’t be suitable for everyone, because known gene mutations determine only a small proportion of cases of Alzheimer’s. But the concept of replacement therapy has been taken up by others. The company Alkahest, based in San Carlos, California, has completed a small clinical trial testing whether factors in the blood of young people could replace those lost in the ageing process7.
Some researchers have found success with lower-tech methods. For example, a large, carefully controlled trial showed that 18 months of either aerobic exercise or stretching stalled cognitive decline in people with mild cognitive impairment, underlying the value of maintaining an exercise regime during pharmacological treatment.
It is too early to say which, if any, of these potential new therapies will pan out. Most researchers think that treatment will need to be personalized: individuals at different disease stages will need different therapies. “It’s great to see so many plausible approaches being pursued,” says Aisen. “But we have a long way to go.”
Still, the range of options going through clinical testing is encouraging, says Sperling. “And our new glimmer of success is moving us forward.”






More News
Daily briefing: Why exercise is good for us
Daily briefing: Orangutan is first wild animal seen using medicinal plant
Old electric-vehicle batteries can find new purpose — on the grid