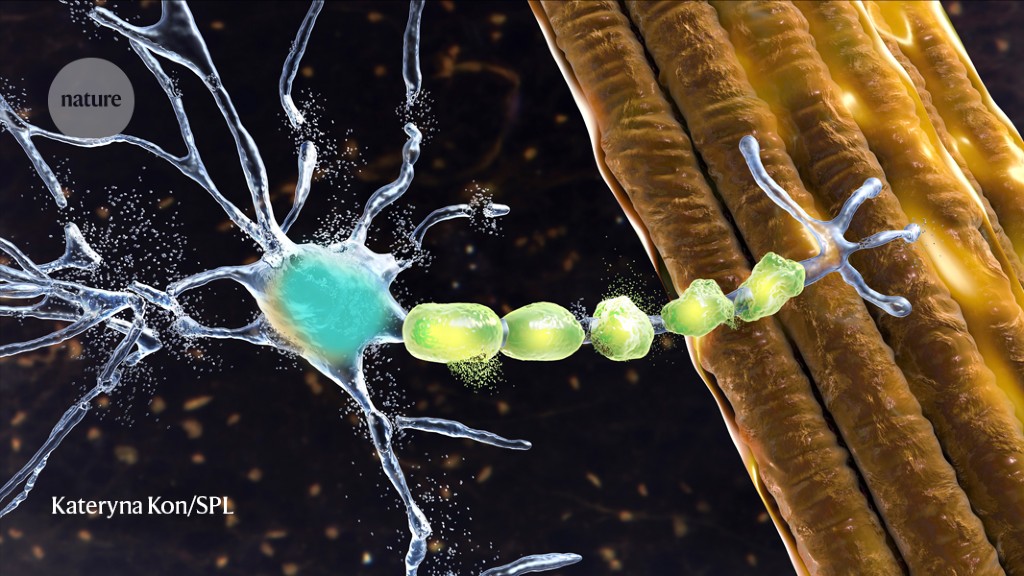
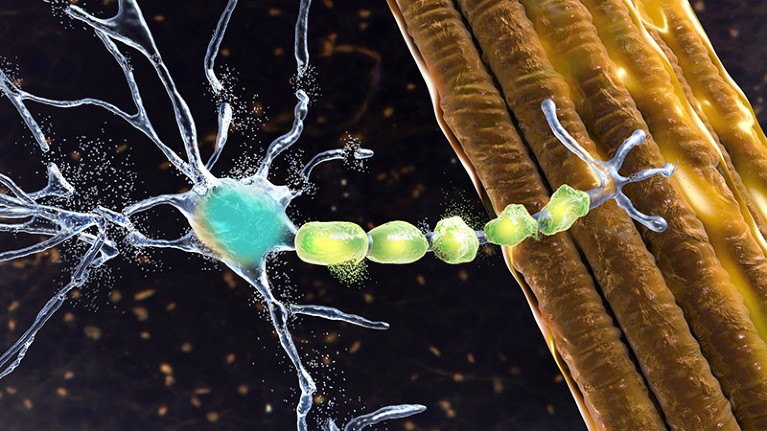
In people with amyotrophic lateral sclerosis (ALS), motor neurons, which help to send commands from the brain to muscle cells, become damaged, as depicted in this artist rendering.Credit: Kateryna Kon/Science Photo Library
The US Food and Drug Administration (FDA) is set to rule soon on the approval of a new drug for a rare form of amyotrophic lateral sclerosis (ALS). The hotly anticipated decision is expected to signpost the agency’s vision for neurological drugs — and its willingness to be flexible in the regulation of these therapeutics.
Genetic therapies offer new hope against incurable brain diseases
People with the disease desperately need new treatments, because they face a degenerative condition that causes neuronal death and typically leads to fatal respiratory failure within three years of symptoms appearing1. Tofersen, developed by the biotechnology firms Biogen in Cambridge, Massachusetts, and Ionis Pharmaceuticals in Carlsbad, California, did not slow patients’ decline in a phase III trial2. However, some say the trial was too short, and point out that there were signs of possible benefit, such as a reduction in a biomarker of neuronal damage and death called neurofilament light chain (NFL). Because of this, Biogen has asked the FDA to approve the drug on an ‘accelerated’ basis, to fast-track it to patients with a guarantee that future trial data will determine whether it works.
If approved, tofersen will become the latest example of the agency’s evolving approach to neurological drug development, which could boost industry investment in brain diseases. A vote of confidence for the drug would also supercharge interest in using NFL as a tool to measure brain health and to test drugs in future. “This could be the start of a new era,” says Valentina Bonetto, a neuroscientist at the Mario Negri Institute for Pharmacological Research in Milan, Italy.
Nature looks at how this decision, due by 25 April, might change the landscape for neurological drugs.
Will the FDA approve tofersen?
In March, the FDA convened a panel to discuss the tofersen data set. Its nine independent advisers rallied behind accelerated approval for the drug, voting unanimously that the available evidence supports a “reasonably likely” chance that tofersen will help people with SOD1 ALS. This rare disease is caused by genetic mutations that affect the protein SOD1, leading it to form toxic clumps in motor neurons in the brain, brainstem and spinal cord. The agency usually follows the recommendations of its advisory committee.
Will the FDA change how it vets drugs following the Alzheimer’s debacle?
Tofersen, an antisense drug, binds to SOD1 mRNA and slows production of the protein. By reducing the build-up of the neurotoxin, tofersen aims to spare motor neurons, and help people to live stronger, longer lives.
Biogen tested tofersen with its phase III VALOR trial2, giving either tofersen or a placebo to 108 people with SOD1 ALS. At the end of the 6-month trial, the drug was evaluated using the ALSFRS-R, a questionnaire that tracks changes in physical function over time. According to this, tofersen recipients fared no better than placebo recipients. However, the drug did lower levels of both SOD1 and NFL. Moreover, the findings of an open-label extension of the trial, in which all of the participants were offered tofersen, indicated that six months might have been too short a period to see an effect.
The FDA’s advisers cited this “constellation of evidence” as a reason to support accelerated approval. But they did not support full approval, voting 5 to 3 — with 1 abstention — against it. Full approval would require fewer or no follow-up studies of the drug, which some of the panellists think are crucial. “Does the provided data in its entirety … provide a convincing level of effectiveness?” asked Tanya Simuni, a neurologist at Northwestern Medicine in Chicago, Illinois. “The short answer is no. … We need more data.”
What does this mean for future neurological drugs?
The FDA’s willingness to review and discuss the tofersen data highlights its newfound flexibility in neurological drug development. “The fact that they’re willing to listen is a good thing for pharmaceutical companies,” says Robert Baloh, global head of neuroscience at Novartis in Basel, Switzerland. “Companies look at this and think, ‘Oh, we should be operating in that space because we’re going to have more chances of being successful’.”
The tofersen deliberations also highlight the rise of NFL as a drug-development tool.
NFL is a rod-like protein that functions as internal scaffolding for neurons. When neurons are damaged or die, they shed NFL detritus into the cerebrospinal fluid and the blood. Neuroscientists have long speculated that levels of NFL could be used to measure brain health. After researchers found a way to measure NFL levels in the blood accurately, around a decade ago, use of the marker gained traction in research labs and in clinical trials3.
Source: Data from clinicaltrials.gov
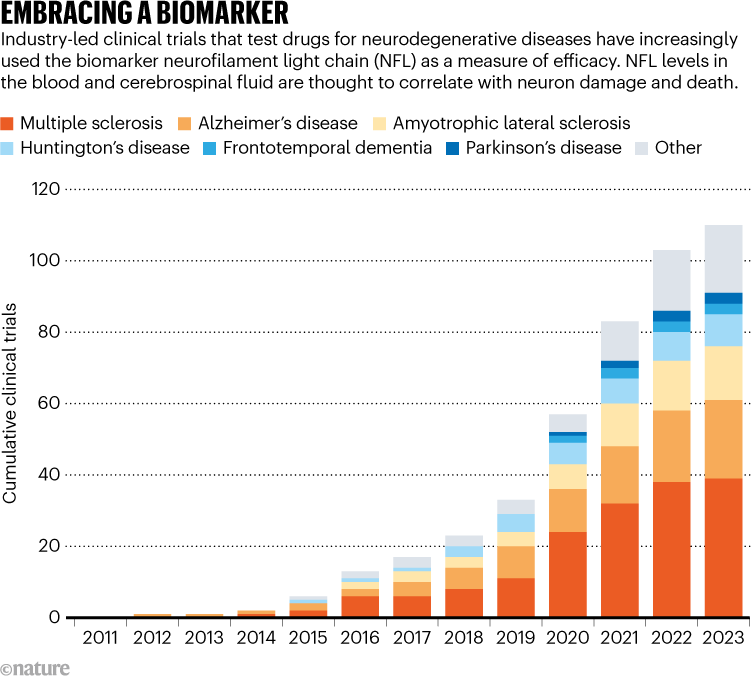
Drug companies have incorporated the tracking of NFL into around 100 clinical trials for various diseases, including Alzheimer’s, Parkinson’s and Huntington’s disease (see ‘Embracing a biomarker’). But its measurement has yet to be used to gain regulatory approval. For Jens Kuhle, a neurologist at University Hospital Basel in Switzerland, an approval for tofersen will “be an important proof of principle for NFL”.
Kuhle is particularly excited by the prospect of using NFL as a tool to work out which multiple sclerosis drugs perform best in which patients, paving the way for ‘personalized’ neurological prescriptions. One day, NFL measurement could be used more broadly still, to identify and track brain damage as part of routine care, he speculates. “I’m pretty convinced that in the future we will be measuring NFL when you go to your family doctor, just like we measure cholesterol.”
Will tofersen ease or re-inflame the debate over the FDA’s use of accelerated approval for neurological drugs?
In 2021, the FDA approved aducanumab, also developed by Biogen, for the treatment of Alzheimer’s disease — despite inconclusive evidence of efficacy and against the recommendation of an advisory panel. That accelerated approval divided the neuroscience community, and an inquiry by US Congress later found that it was “rife with irregularities”. Critics questioned not just the reliability of the data on aducanumab, but also Biogen’s selection of a budget-breaking price tag for the drug.
Landmark Alzheimer’s drug approval confounds research community
The application for tofersen’s approval has important differences — including how many people it will affect. In the United States, Alzheimer’s disease affects more than 6 million people. Just 330 have SOD1 ALS.
These neurodegenerative diseases are “different beasts”, meaning they call for different regulatory maths, says Scott Ayton, a neuroscientist at the Florey Institute of Neuroscience and Mental Health in Melbourne, Australia. More regulatory flexibility is needed for rare diseases, Ayton says, because it is harder to run and replicate trials when patient numbers are low.
But, he adds, giving the green light to tofersen would not be without risks — for patients or the clinical-trials ecosystem. “The unintended consequence of these accelerated approvals is poorly designed and conducted clinical trials,” he says. In the case of tofersen, Biogen designed a phase III trial that was too short to definitively assess the effect of the drug. Regulators, physicians and patients now have to make the most of the inconclusive data set. “If the threshold for approval is lower, industry is going to go after a lower threshold,” Ayton says.
“We did miss the mark,” said Stephanie Fradette, senior medical director at Biogen, when the advisory committee asked about the trial’s design flaws. But because of the rarity of SOD1 ALS, she said the company had a limited data set to guide its trial-design decisions. “Certainly this is not intended to be an excuse, but an acknowledgement of why things were so different from what we had anticipated.”







More News
Editorial Expression of Concern: Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase – Nature
Quantum control of a cat qubit with bit-flip times exceeding ten seconds – Nature
Venus water loss is dominated by HCO+ dissociative recombination – Nature