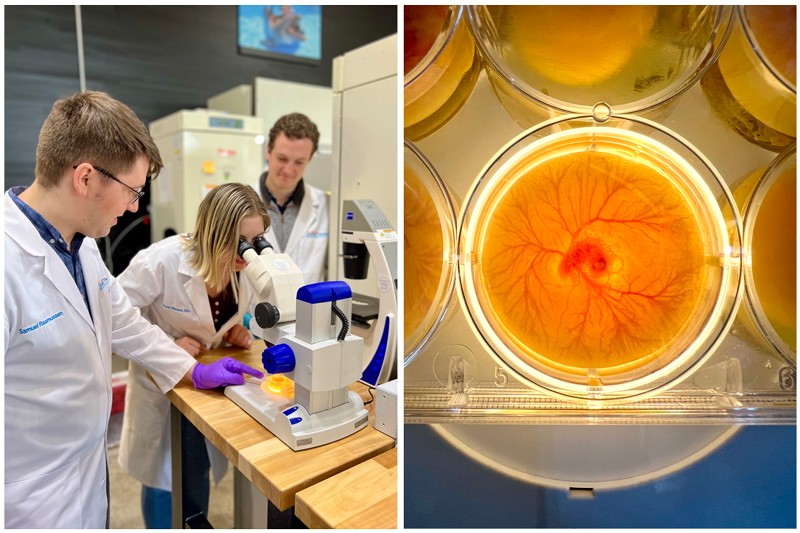
In a single motion, a sliding blade slices the bottoms off six speckled quail eggs, and their yolks plop into a six-well dish.
Quail eggs aren’t common in research, and most biomedical scientists have never seen this guillotine-like device. But at the biotechnology laboratory of the Children’s Cancer Therapy Development Institute (cc-TDI), just outside Portland, Oregon, these eggs are helping researchers to quickly prioritize drug candidates for long, costly mouse studies. Last December, cc-TDI published a description of this platform1 in Scientific Reports — just one example of how the non-profit organization is breaking norms in cancer research as one of the only freestanding research labs focused on developing drugs for paediatric cancer.
Each year, some 400,000 children and adolescents around the world develop cancer. In the United States, cancer kills around 1,800 young people annually, more than all other diseases combined. Yet, since 1978, fewer than a dozen drugs developed specifically for childhood cancers have earned US Food and Drug Administration approval. (On average, 12 drugs for adult cancers reach the market every year.)
It’s a cruel economic conundrum: despite their huge collective impact, individual childhood cancers are rare, and few companies are willing to invest the millions of dollars required to develop a drug with a tiny market. “How on Earth are you going to make money treating 300 kids a year with rhabdomyosarcoma?” asks Charles Keller, scientific director and founder of cc-TDI, referencing a rare type of cancer that forms in muscle and other soft tissues and that affects mostly children.
A physician-researcher with a background in biomedical engineering, Keller launched cc-TDI in 2015 to change that calculus. Partnering with biotech and pharmaceutical companies to vet experimental therapies for clinical testing, the institute has already helped to move a pair of drug candidates into nationwide phase I trials, including one for diffuse intrinsic pontine glioma, a lethal brain tumour for which the survival rate and treatment options have not improved in several decades.
Flexible funding
Federal grants don’t typically fund drug-validation and preclinical-development studies, so researchers aren’t financially incentivized to complete them. A new mechanism, a promising drug target, perhaps a candidate drug — those are the kinds of project that tend to attract government investments.
cc-TDI runs on a lean US$2.5-million budget — of which just $350,000 is supplied by grants from the US National Institutes of Health (NIH). The bulk comes from foundations, families and philanthropy. “It takes just as much work to steward a relationship of trust with a donor as it does to write an NIH grant,” Keller says. One young woman raised more than $980,000 for cc-TDI before dying of rhabdomyosarcoma in March, a month shy of her 21st birthday. A Portland-area philanthropist gave $270,000.
Philanthropy and private donations can fund high-impact preclinical research that would struggle to attract federal dollars. With funding from multiple foundations, for instance, cc-TDI laid the groundwork for repurposing a class of antibiotic compounds called fluoroquinolones to prevent relapses in Ewing sarcoma, a rare cancer for which two drugs have failed clinical trials. Fluoroquinolones could enter clinical trials once cc-TDI secures funding to identify promising compounds and test them in more cell and animal models.
An engineer’s perspective
For tools to get the job done, the institute turns to engineers. Noah Berlow came on board in 2015 after obtaining an electrical engineering PhD at Texas Tech University in Lubbock. In his research, Berlow used applied maths and artificial intelligence to find cancer therapies, as part of a collaboration between Keller, then at the Oregon Health & Science University in Portland, and Ranadip Pal, an electrical engineer and Berlow’s thesis adviser at Texas Tech.
At cc-TDI, engineers and biologists work together to analyse drug-testing results alongside DNA- and RNA-sequencing data of tumours — resulting in massive data sets. Berlow says the engineer’s role is to help make sense of them. When intriguing features in the data emerge, biologists can work out what they mean. Although engineers rely on biologists’ expertise to contextualize what might appear as a smattering of stray data points, an engineer’s scant biomedical knowledge — and their fresh perspective — can prove advantageous.
“When you start making assumptions that you know how things work, cancer has a way of turning that on its head,” Berlow says.
Berlow co-developed an automated screening tool that ranks by diagnosis likelihood all the possible cancers a person could have. This helps pathologists to decide quickly which confirmatory tests to conduct, and could prove especially useful in rural areas and developing countries that lack pathologists2. Trained on 424 tissue slides of sarcoma tumours, the model is more than 88% effective at detecting all tested sarcoma subtypes.
Another engineer, Samuel Rasmussen, joined cc-TDI fresh out of university, where he studied mechanical engineering. Rasmussen put himself through Portland State University in Oregon by working nights at a local distribution warehouse of the farm-equipment manufacturer John Deere. He was part of a team of undergraduates doing a senior research project in early 2016 under Keller’s supervision. Their charge: create a device to crack eggs without breaking the yolk.
The team’s design didn’t work, but Rasmussen kept tinkering. Experimenting over a mixing bowl at the student union, he determined that removing the bottom of the egg with a knife could release the yolk unscathed. Within days, Rasmussen created a working prototype. Keller offered him a summer internship — and, six months later, hired him full time.
Rasmussen was first author of the Scientific Reports paper1, in which he and colleagues placed drug-treated tumour cells onto shell-free quail embryos growing in lab dishes, providing a quick and inexpensive way to screen drugs on living tissue. Data from an 11-day quail-egg assay, which uses up to 200 eggs per screening at around 35 cents an egg, agreed with mouse data, even when results from mouse and lab-dish experiments differed, Keller says. That suggests the quail-egg system could be used to reliably select candidates for testing in studies using mice implanted with human tumours. Those mouse studies take ten or more weeks and cost tens of thousands of US dollars.
Because mouse studies “are really expensive and time-consuming”, says Maya Ridinger, a biologist at Cardiff Oncology, a biotech firm based in San Diego, California, scientists can investigate only a limited number of compounds, doses and models. Cardiff Oncology is working with cc-TDI to test onvansertib, a drug designed to treat a childhood liver cancer called hepatoblastoma. “I think the quail-egg system is a great opportunity,” says Ridinger.
Mission minded
Rasmussen’s work was funded by a John Deere dealership owner who gave money to cc-TDI after losing a niece to childhood cancer. The John Deere link was coincidental, but Keller has a knack for rallying diverse people to a singular mission — getting drugs into paediatric-cancer trials. In addition to nurturing connections with families and funders, Keller maintains close ties with pharmaceutical collaborators and groups that run clinical trials, to focus cc-TDI’s preclinical work on what is potentially translatable. “You probably can’t overstate that, because we’ve been curing cancer in mice for many years,” says Douglas Hawkins, a paediatric haematologist-oncologist at Seattle Children’s in Washington. Hawkins, who chairs the Children’s Oncology Group, a federally funded clinical-trial network that is conducting one of the cc-TDI trials, adds: “Trying to take whatever we’ve learnt in the lab and apply it to humans, that’s been one of the harder things.”
Since 2004, the US National Cancer Institute has funded a preclinical research programme to evaluate compounds — mostly pharmaceutical drugs previously developed for adult cancers — for inclusion in paediatric cancer trials. For the five-year funding cycle that began in July 2021, the Pediatric Preclinical In Vivo Testing Consortium (PIVOT) provided $5 million per year to a coordinating centre and seven research teams to test specific compounds in models in the lab. Among more than 140 drugs studied by the programme, only a few have proceeded to clinical testing, says paediatric oncologist and PIVOT director Malcolm Smith.
At cc-TDI, Keller aims to beat those odds by focusing on team diversity and his belief that a great scientist can come from anywhere.
Andy Woods, for example, is a former tile contractor who left his business and moved his family to Oregon in 2017 to work at cc-TDI as a senior research associate. In October 2021, he published a paper3 on his daughter’s kidney cancer, Wilms’ tumour, describing how he and his colleagues used genomics to identify drug candidates for a subtype of the disease that responds poorly to standard therapies.
Another recent addition to cc-TDI is Tim Brown, a former vice-president of biotech company Genentech, based in South San Francisco, California. In 2015, Brown’s 20-year-old son died from Duchenne muscular dystrophy. Brown connected with Keller through a cc-TDI research assistant who was part of the team that helped his son, then an undergraduate at the University of Portland, with eating, dressing and daily tasks as his disease progressed. Brown began volunteering at cc-TDI last October to honour his son’s memory by applying his supply-chain and manufacturing expertise to projects such as automating the quail-egg assay.
The multidisciplinary team and culture of innovation were a big draw, Brown says. “There’s really good connection between us folks who have a few years of experience in different industries, and these young scientists.”
By casting a wide net, the institute “was, and always is, an experiment”, Keller says. Its junior board of directors, which helps to plan local events and fundraising efforts, is open to young people aged 7 to 17. It has former vice-presidents of the semiconductor manufacturer Intel on its board of directors. And it hosts annual summer ‘nanocourses’, weeklong crash courses in the basics of childhood cancers, drug development and clinical trials, to train members of the public to liaise between cancer researchers and the community. “This is a grass-roots cause,” Keller concludes, “where a few people who care a lot about a rare condition come together because they’re driven by the mission.”









More News
Full-colour 3D holographic augmented-reality displays with metasurface waveguides – Nature
Observation of Nagaoka polarons in a Fermi–Hubbard quantum simulator – Nature
Lithium tantalate photonic integrated circuits for volume manufacturing – Nature