Barriers around the brain and spinal cord of the central nervous system (CNS) protect neuronal cells from the changeable milieu of the bloodstream by controlling movement of molecules and cells between the blood and the CNS. These barriers also ensure that the CNS can be kept under surveillance by certain immune cells, but restrict the access of blood-derived immune cells and molecules to specific compartments at the border of the CNS1. Writing in Science, Cugurra et al.2 and Brioschi et al.3 report that the dura mater, a tissue layer around the outermost barrier of the CNS, sources a private immune protection from nearby bone marrow.
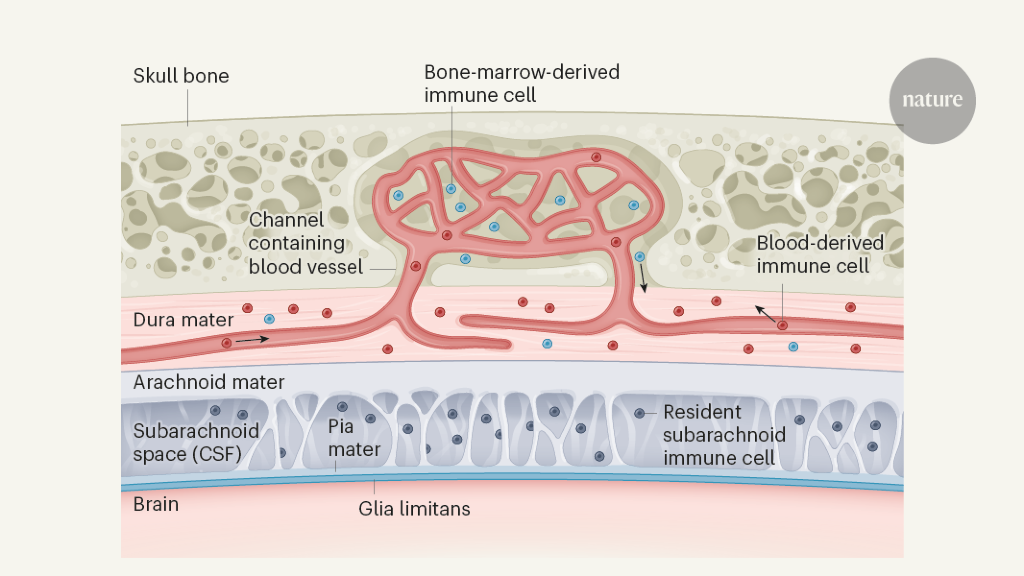
Encasing the brain and the spinal cord are three meningeal membranes1,4 (Fig. 1). The outermost membrane, the dura mater, lacks a blood–brain barrier, and so the entry of blood-derived components, including immune cells, into this layer is unrestricted1,4. The arachnoid mater is attached to the inner surface of the dura mater. Between the arachnoid mater and the innermost meningeal layer, the pia mater, is the subarachnoid space, which contains cerebrospinal fluid (CSF) and resident immune cells that enter during embryonic development5. The arachnoid mater acts as a blood–CSF barrier between the dura mater and the subarachnoid space. The pia mater lies directly on top of the glia limitans, a thin layer of extracellular-matrix material and cell-protrusion endings at the surface of the CNS tissue4. The anatomy of the meningeal layers has been likened to the defences around a medieval castle, with two walls (the arachnoid barrier and the glia limitans) bordering a guard-patrolled moat (the subarachnoid space and its immune cells)6.
The two new studies focused on different subsets of immune cells, namely, myeloid cells of the innate branch of the immune system (which recognizes stereotypical changes characteristic of infection)2 and B cells of the adaptive immune system (which responds to and remembers specific foreign invaders)3. The authors attached the circulatory system of one mouse, in which these subsets of immune cells were fluorescently tagged, to that of a second, untreated, mouse, and made the surprising finding that fewer tagged cells than untagged cells were observed in the dura mater of the second mouse. This finding suggests that a considerable proportion of immune cells in the dura mater do not arrive from the bloodstream, but instead originate from the bone marrow in the skull and the vertebrae of the spine. This shortcut is made possible by the cells crawling along the outside of blood vessels inside small, bony channels, identified previously7, between the bone marrow and the dura mater (Fig. 1). Thus, the dura mater sources a private immune protection right outside the outer CNS barrier (the arachnoid mater) from adjacent bone marrow through a previously unrecognized route.
Analysis of gene expression and other characteristics of the individual dura mater immune cells supported the idea that these bone-marrow-derived immune cells are programmed to ensure CNS health, whereas those arriving from the blood tend to be pro-inflammatory and thus more ready to fight potential infections. Moreover, with ageing, increasing numbers of blood-derived immune cells were observed in the dura mater, suggesting a shift in CNS-border immune protection.
Unexpectedly, Cugurra et al.2 found a large number of a type of bone-marrow-derived immune cell called granulocytes in the dura mater. Granulocytes are not typically resident in tissue: they are short-lived, circulate in the blood and usually infiltrate tissue only during acute inflammation. However, the authors found these cells in the apparently uninflamed dura mater, and, indeed, granulocytes have been observed in healthy meninges previously6.
Therefore, the bone-marrow-derived granulocytes might be a special subset of granulocytes with functions different from those of the cells from the blood. This finding is highly relevant to stroke caused by insufficient oxygen reaching the brain, because the presence of granulocytes in the meninges is a major hallmark of such an event8,9, and yet blocking granulocyte infiltration from the blood had no effect on the outcome of stroke in clinical trials10. Previous work7 showed that the oxygen-deprived brain can recruit granulocytes directly from the skull bone marrow to the brain tissue by way of the same bony channels described in the present studies.
The signals that recruit cells from the bone marrow to the dura mater remain to be identified. Cugurra et al. and Brioschi et al. propose a role for the chemokine molecule CXCL12 and its cell-surface receptor CXCR4. However, CXCL12 usually promotes immune-cell retention in the bone marrow11, and blocking CXCR4 would release these bone-marrow cells into the blood11. Thus, these roles of CXCL12 and CXCR4 are difficult to reconcile with a role in direct immune-cell recruitment to the dura mater. One possibility is that blocking CXCR4 on bone-marrow granulocytes might enable these cells to sense gradients of the inflammatory chemokines CCL2 and CCL8, which Cugurra and colleagues found to be highly expressed in the dura mater.
Another indication that the dura mater might provide the CNS with a special form of immune surveillance was that it contains a substantial number of immature B cells3 expressing CXCR4, similar to those in the bone marrow. This suggests that the bone marrow outsources part of its role in B-cell maturation to the dura mater. Indeed, cells of the dura mater were found to produce CXCL12, thus providing a bone-marrow-like environment for these immature B cells.
The signals that drive movement of premature B cells from the bone marrow to the dura mater remain to be identified. The authors speculate that the brain and spinal cord might program immune cells coming from adjacent bone-marrow niches to provide CNS-tailored immune protection. A key remaining question is how the CNS communicates with the immune cells in the dura mater or the bone marrow in the skull and vertebrae, because they are on different sides of the arachnoid barrier, which establishes a barrier between the CNS and the changing blood milieu.
Notably, in this context, the dura mater sends many blood vessels into the skull bone9. And, intriguingly, both studies found high levels of myeloid cells and B cells in the dura mater that encases the dural sinus (the large central vein that drains blood, and probably CSF, from the brain), particularly at sites where blood vessels from the CNS, and the subarachnoid space, join the dural sinus. Previous work from some of the authors of the current studies suggested that the dura mater along the dural sinus constitutes a special neuroimmune interface12. It is thus tempting to speculate that the signals driving the recruitment of immune cells from the bone marrow to the dura mater, and potentially into the CNS itself, do not cross the arachnoid barrier, but instead move along the walls of blood vessels that form a bridge between brain barriers such as the arachnoid barrier and the glia limitans.
Future research should determine whether skull and vertebra bone marrow are different from bone marrow elsewhere. Furthermore, how do the functions of bone-marrow-sourced immune cells in the dura mater compare with those of the immune cells that keep the subarachnoid space under surveillance? The signals attracting immune cells to the dura mater from the bone marrow, as opposed to from the blood, must also be explored. And how do the bone-marrow-sourced immune cells in the dura mater interact with those from the blood?
The channels connecting the skull and vertebra bone marrow with the dura mater also exist in humans7, suggesting that observations of immune-cell migration and function in mice might translate to humans. Moreover, because the dura mater lacks a blood–brain barrier, immune cells there could be more easily therapeutically targeted than immune cells in the subarachnoid space or in CNS tissue itself. Understanding the precise role of these dura mater immune cells, the signals that control them and how they contribute to CNS immunity could therefore open up entirely new avenues for the design of treatments for disorders involving CNS inflammation.
Competing Interests
The author declares no competing interests.






More News
Star Formation Shut Down by Multiphase Gas Outflow in a Galaxy at a Redshift of 2.45 – Nature
Garden-variety fungus is an expert at environmental clean-ups
Air-travel climate-change emissions detailed for nearly 200 nations